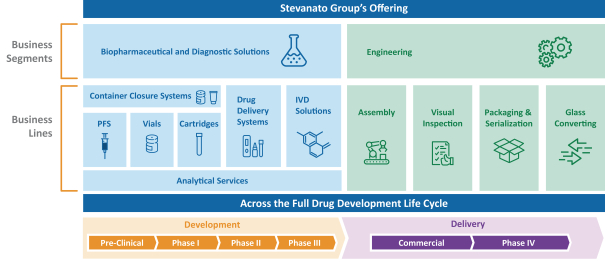
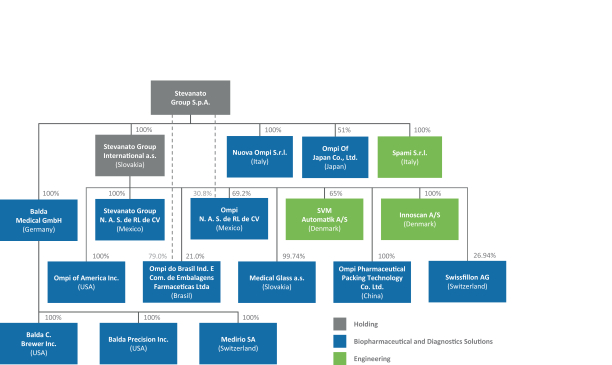
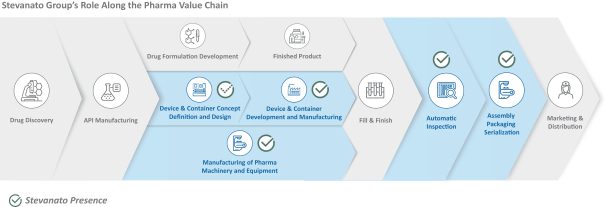
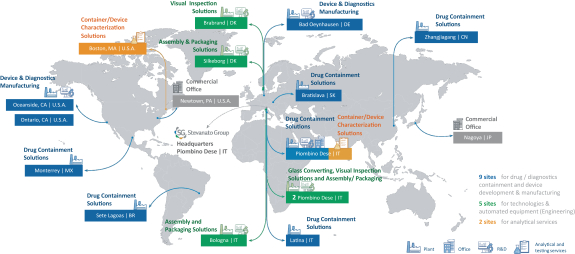
The information in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS
SUBJECT TO COMPLETION, DATED , 2021
STEVANATO GROUP S.P.A.

Ordinary shares
This is an initial public offering of ordinary shares of par value €1,000.00 per share in the capital of Stevanato Group S.p.A. (“Shares”) by Stevanato Group S.p.A. The selling shareholder identified in this prospectus is offering an additional Shares.
We currently estimate that the initial public offering price will be between $ and $ per Share. Prior to this offering, there has been no public market in the United States, or the U.S., for our Shares. Application has been made for the listing of our Shares on the (“ ”) under the ticker STVN. We believe that upon completion of the offering contemplated by this prospectus, we will meet the standards for listing on the main exchange.
Following the completion of this offering, our outstanding share capital will consist of Class ordinary shares and Class multiple voting shares. Stevanato Holding S.r.l. will beneficially own all of our issued Class multiple voting shares and will be able to exercise % of the total voting power of our issued and outstanding share capital immediately following the completion of this offering. Holders of Class ordinary shares will be entitled to one vote per share, while holders of Class multiple voting shares will be entitled to three votes per share. Upon any sale, transfer, assignment or disposition of any Class multiple voting shares by a holder thereof to a non-affiliate of such holder, each of such Class multiple voting shares will be automatically and immediately converted into one Class ordinary share.
Following the completion of this offering, we will be a “controlled company” within the meaning of the corporate governance rules of the because Stevanato Holding S.r.l. will beneficially own % of the total voting power of our then outstanding share capital, assuming the underwriters do not exercise their over-allotment option, or % of our then outstanding share capital if the underwriters exercise their over-allotment option in full. See “Principal and Selling Shareholder” beginning on page 113.
We are both an “emerging growth company” and a “foreign private issuer” as defined under U.S. federal securities laws and as such, will be eligible for reduced public company reporting requirements for this prospectus and future filings.
Investing in our shares involves risks. see “Risk Factors” beginning on page 20.
Neither the United States Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities, or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
PRICE US$ per Share
| Price
to |
Underwriting |
Proceeds |
Proceeds
to |
|||||||||||||
| Per ordinary share |
$ | $ | $ | $ | ||||||||||||
| Total |
$ | $ | $ | $ | ||||||||||||
| (1) | See the section entitled “Underwriting” for additional disclosure regarding underwriting compensation payable by us. |
To the extent that the underwriters sell more than Shares, the selling shareholder has granted the underwriters the right to purchase up to an additional Shares at the same price as the Shares offered through this prospectus, for days after the date of this prospectus.
The underwriters expect to deliver the Shares against payment in U.S. dollars in New York, New York on or about , 2021.
| MORGAN STANLEY | BofA SECURITIES | JEFFERIES |
Prospectus dated , 2021.