UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
☐ |
|
OR |
|
☒ |
|
|
For the fiscal year ended |
OR |
|
☐ |
|
OR |
|
☐ |
|
|
Date of event requiring this shell company report |
Commission File Number:
(Exact name of registrant as specified in its charter)
Republic of
(State or other jurisdiction of incorporation or organization)
Telephone: +39 049 931811
(Address, including zip code, and telephone number, including area code, of principal executive offices)
Telephone:
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
Title of Each Class |
Trading Symbol |
Name of Each Exchange on Which Registered |
The |
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report:
As of the date of this annual report on Form 20-F, there were
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes ☐
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such a shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
Accelerated filer ☐ |
Non-accelerated filer ☐ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards † provided pursuant to Section 13(a) of the Exchange Act.
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant's executive officers during the relevant recovery period pursuant to Section 250.10D-1(b).
☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S.GAAP ☐ |
Other ☐ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the Registrant has elected to follow:
Item 17 ☐ Item 18 ☐
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ☐
(APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY PROCEEDINGS DURING THE PAST FIVE YEARS)
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court.
Yes ☐ No ☐
TABLE OF CONTENTS
|
||
ITEM 1. |
1 |
|
A. |
1 |
|
B. |
1 |
|
C. |
1 |
|
ITEM 2. |
1 |
|
A. |
1 |
|
B. |
1 |
|
ITEM 3. |
1 |
|
A. |
1 |
|
B. |
1 |
|
C. |
1 |
|
D. |
1 |
|
ITEM 4. |
37 |
|
A. |
37 |
|
B. |
38 |
|
C. |
68 |
|
D. |
68 |
|
ITEM 4A. |
68 |
|
ITEM 5. |
69 |
|
A. |
69 |
|
B. |
80 |
|
C. |
92 |
|
D. |
92 |
|
E. |
92 |
|
ITEM 6. |
93 |
|
A. |
93 |
|
B. |
96 |
|
C. |
102 |
|
D. |
107 |
|
E. |
107 |
|
f. |
disclosure of a registrant's actionto recover erroneously awarded compensation |
107 |
ITEM 7. |
107 |
|
A. |
107 |
|
B. |
108 |
|
C. |
111 |
|
ITEM 8. |
112 |
|
A. |
112 |
|
B. |
112 |
|
ITEM 9. |
112 |
|
A. |
112 |
|
B. |
112 |
|
C. |
112 |
|
D. |
112 |
|
E. |
112 |
|
F. |
112 |
|
ITEM 10. |
112 |
|
A. |
112 |
|
B. |
114 |
|
C. |
135 |
|
D. |
135 |
|
E. |
136 |
|
F. |
148 |
|
i
G. |
148 |
|
H. |
148 |
|
I. |
149 |
|
J. |
149 |
|
ITEM 11. |
149 |
|
ITEM 12. |
149 |
|
A. |
149 |
|
B. |
149 |
|
C. |
149 |
|
D. |
149 |
|
150 |
||
ITEM 13. |
150 |
|
ITEM 14. |
MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS |
150 |
ITEM 15. |
150 |
|
ITEM 16. |
152 |
|
ITEM 16A. |
152 |
|
ITEM 16B. |
153 |
|
ITEM 16C. |
153 |
|
ITEM 16D. |
153 |
|
ITEM 16E. |
PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS |
154 |
ITEM 16F. |
154 |
|
ITEM 16G. |
154 |
|
ITEM 16H. |
155 |
|
ITEM 16I. |
DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
155 |
item 16.J. |
155 |
|
item 16.k. |
155 |
|
157 |
||
ITEM 17. |
157 |
|
ITEM 18. |
157 |
|
ITEM 19. |
157 |
|
ii
Throughout this annual report, unless the context otherwise requires, references to “Stevanato Group S.p.A.”, “Stevanato”, the “Company”, “we”, “us”, “Group”, “our” and words of similar import refer to Stevanato Group S.p.A. and its consolidated subsidiaries.
Unless otherwise indicated, all references to “€”, “EUR” and “Euro” in this annual report are to, and amounts are presented in, euros. All references to “US$” and “$” are to U.S. Dollars.
Financial Statements
We present in this annual report the audited consolidated financial statements as of December 31, 2023 and 2022, and for the years ended December 31, 2023, 2022 and 2021. These financial statements were prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board (IFRS). The consolidated financial statements and the notes to the consolidated financial statements are referred to collectively as the “Consolidated Financial Statements”.
All references herein to “our financial statements” or “our consolidated financial statements”, are the Consolidated Financial Statements included elsewhere in this annual report.
Our fiscal year ends on December 31. References in this annual report to a fiscal year, such as “fiscal year 2023,” relate to our fiscal year ended on December 31 of that calendar year.
As of the date of this annual report, our authorized share capital is €21,698,480.00 divided into 295,540,036 shares without par value, including 34,870,467 ordinary shares and 260,669,569 Class A shares, of which 30,073,093 shares are held in treasury. On March 4, 2021, the shareholders’ meeting approved a share split following which the then existing 20,002 shares have been split into a total of 100,010,000 ordinary shares with no par value, without changing the amount of the share capital. On July 1, 2021 the shareholders’ meeting approved a further share split following which all the existing 100,010,000 shares have been split into a total of 272,427,240 shares in the ratio of 2.724 new shares post-split for each share outstanding prior to the share split. In connection with the split that occurred on July 1, 2021, all of the ordinary shares held by Stevanato Holding S.r.l. and the ordinary shares held in treasury were converted into Class A shares.
On July 20, 2021 we completed our initial public offering, at completion of which 22,400,000 ordinary shares were offered by us and 9,600,000 ordinary shares were offered by Stevanato Holding S.r.l.. On August 18, 2021 the underwriters further purchased 712,796 newly issued ordinary shares from us and 305,484 ordinary shares from Stevanato Holding S.r.l.
On August 30, 2023, for the purpose of granting of ordinary shares to the beneficiaries of the Restricted Stock Grant Plan Stevanato Group S.p.A. 2021-2022, 364,139 Class A shares held in treasury were converted into ordinary shares and, thereafter, granted to the relevant beneficiary; as a result of such conversion, our share capital (divided into a total of 295,540,036 Shares) included 34,467,144 ordinary shares and 261,072,892 Class A shares.
On September 7, 2023, for the purpose of granting of ordinary shares to directors and employees of the Company or of its subsidiaries as compensation in kind or as benefit, bonus or other premium or incentive (also in execution of the Restricted Stock Grant Plan Stevanato Group S.p.A. 2021-2022), 403,323 Class A shares held in treasury were converted into ordinary shares and, thereafter, granted to the relevant beneficiary; as a result of such conversion, our share capital (divided into a total of 295,540,036 Shares) included 34,870,467 ordinary shares and 260,669,569 Class A shares.
On October 4, 2023, pursuant to an extraordinary shareholders’ meeting, the board of directors, pursuant to Article 2443 of the Italian Civil Code, was delegated the authority to increase the share capital in cash, on one or more occasions, also on a divisible basis pursuant to Article 2439 of the Italian Civil Code, within the term of October 4, 2028, for a maximum amount of €350,000,000.00, including any share premium, by issuing ordinary shares, with no par value, carrying full dividend rights, in one or more tranches, to be offered by excluding the existing shareholders’ pre-emptive right pursuant to Article 2441, Paragraph 4, second sentence, of the Italian Civil Code (and, therefore, within the limit of 10% (ten per cent) of the overall number of Company’s Shares currently outstanding).
iii
Market Share and Other Information
This annual report contains data related to economic conditions in the market in which we operate. The information contained in this annual report concerning economic conditions is based on publicly available information from third-party sources that we believe to be reasonable. Market data and certain industry forecast data used in this annual report were obtained from internal reports and studies, where appropriate, as well as estimates, market research, publicly available information and industry publications. We obtained the information included in this annual report relating to the industry in which we operate, as well as the estimates concerning market shares, through internal research, public information and publications on the industry prepared by official public sources.
There are a number of studies that address either specific market segments, or regional markets, within our industry.
We have reviewed and analyzed data collected by, among others, IQVIA, Alira Health, Roots Analysis, Markets and Markets Research Pvt Ltd., Grand View Research and Evaluate MedTech and Global Data UK Ltd (“Global Data”). However, given the rapid changes in our industry and the markets in which we operate, no industry research that is generally available covers all of the trends we view as key to understanding our industry and our place in it as providers of drug containment, drug delivery and diagnostic solutions for the pharmaceutical, biotechnology and life sciences industries.
Due to the evolving nature of our industry and competitors, we believe that it is difficult for any market participant, including us, to provide precise data on the market or our industry. However, we believe that the market and industry data we present in this annual report provide accurate estimates of the market and our place in it. Forecasts and other forward-looking information obtained from these sources are subject to the same qualifications and uncertainties as other forward-looking statements in this annual report. We have no reason to believe any of this information or these reports are inaccurate in any material respect and believe and act as if they are reliable. In addition, the data that we compile internally and our estimates have not been verified by an independent source. None of the publications, reports or other published industry sources referred to in this annual report were commissioned by us or prepared at our request. Except as disclosed in this annual report, we have not sought or obtained the consent of any of these sources to include such market data in this annual report.
Rounding
Certain numerical figures, including financial data presented in millions and thousands, have been subject to rounding adjustments and, as a result, the totals of the data may vary slightly from the actual arithmetic totals of such information. In addition, as a result of such rounding, the totals of certain financial information presented in tabular form may differ from the information that would have appeared in such totals using the unrounded financial information.
The financial data in the "ITEM 5. Operating and Financial Review and Prospects" is presented in millions of Euro, while the percentages presented are calculated using the underlying figures in Euro.
Trademarks, Service Marks and Trade Names
We have proprietary rights to trademarks used in this annual report that are important to our business, many of which are registered under applicable intellectual property laws. Solely for convenience, the trademarks, service marks, logos and trade names referred to in this annual report are without the ® and ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensors to these trademarks, service marks and trade names. This annual report contains additional trademarks, service marks and trade names of others, which are the property of their respective owners. All trademarks, service marks and trade names appearing in this annual report are, to our knowledge, the property of their respective owners. We do not intend our use or display of other companies’ trademarks, service marks, copyrights or trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
iv
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Forward-looking statements include statements concerning plans, objectives, goals, strategies, future events or performance, and underlying assumptions and other statements, which are other than statements of historical or present facts or conditions. These forward-looking statements are contained principally in the sections entitled “Risk Factors”, “Operating and Financial Review and Prospects” and “Business.” These statements relate to events that involve known and unknown risks, uncertainties and other factors, including those listed under “Risk Factors,” which may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
In some cases, these forward-looking statements which reflect our current views with respect to future events and financial performance. The words “believe,” “anticipate,” “intend,” “estimate,” “forecast,” “project,” “plan,” “potential,” “may,” “should,” “expect”, “envisage”, and similar expressions identify forward-looking statements. Forward-looking statements contained in this annual report include, but are not limited to, statements about:
The forward-looking statements in this document are based upon various assumptions, many of which are based, in turn, upon further assumptions, including, without limitation, management’s examination of historical operating trends, data contained in our records and other data available from third parties. Although we believe that these assumptions are reasonable, because these assumptions are inherently subject to significant uncertainties and contingencies that are difficult or impossible to predict and are beyond our control, we cannot assure you that we will achieve or accomplish these expectations, beliefs or projections.
In addition to these important factors and matters discussed elsewhere in this annual report, important factors that, in our view, could cause actual results to differ materially from those discussed in the forward-looking statements include:
v
We caution readers of this annual report not to place undue reliance on these forward-looking statements, which speak only as at their dates. We undertake no obligation to update any forward-looking statement or statements to reflect events or circumstances after the date on which such statement is made or to reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible for us to predict all of these factors. Further, we cannot assess the impact of each such factor on our business or the extent to which any factor, or combination of factors, may cause actual results to be materially different from those contained in any forward-looking statement.
vi
PART I
ITEM 1. Identity of Directors, Senior Management and Advisers
Not applicable.
Not applicable.
Not applicable.
ITEM 2. Offer Statistics and Expected Timetable
Not applicable.
Not applicable.
ITEM 3. Key Information
Not applicable.
Not applicable.
Our business, financial condition, results of operations and liquidity can suffer materially as a result of any of the risks described below. While we have described all of the risks we consider material, these risks are not the only ones we face. We are also subject to the same risks that affect many other companies, such as technological obsolescence, labor relations, geopolitical events, climate change and risks related to the conducting of international operations. Additional risks not known to us or that we currently consider immaterial may also adversely impact our businesses. Our businesses routinely encounter and address risks, some of which may cause our future results to be different—sometimes materially different—than we presently anticipate.
Summary
The following summarizes some, but not all, of the risks provided below. Please carefully consider all of the information discussed in this Item 3.D. “Risk Factors” in this annual report on Form 20-F for a more thorough description of these and other risks.
Risks Relating to our Business and Industry
1
2
Risks Relating to our Intellectual Property
Risks Relating to our Shares
3
Risk Factors
Risks Relating to our Business and Industry
Our product offerings are highly complex, and, if our products do not satisfy applicable quality criteria, specifications and performance standards, we could experience lost sales, delayed or reduced market acceptance of our products, increased costs and damage to our reputation.
Most of our products are highly exacting and complex due to their use for containment and injection of biologic drugs and vaccines. Providing high-quality products that deliver specificity, sensitivity and consistency, together with extensive product validation data is a fundamental driver of customer loyalty and our reputation with life sciences researchers. Our operating results depend on our ability to execute and, when necessary, improve our global quality control systems, including our ability to effectively train and maintain our employees with respect to quality control. A failure of our global quality control systems could result in problems with facility operations or preparation or provision of defective or non-compliant products which could ultimately cause harm to the final user. In each case, such problems could arise for a variety of reasons, including equipment malfunction, failure to follow specific protocols and procedures, problems with critical materials and components, failure by one or more of our suppliers to meet our quality requirements, or environmental factors and damage to, or loss of, manufacturing operations. Such problems could affect production of a particular batch or series of batches of products, requiring the destruction of such products or a halt of facility production altogether. Although we currently hold an insurance policy that covers liabilities for defective products and product recalls in amounts we believe to be adequate for our business, our coverage may not be adequate to insure against all product liability claims that may arise which may be particularly high in case failure of our products to meet the appropriate quality standards which may cause product recalls or damages to our customers or ultimate users. As a result of this, product defect claims or product recalls may have a material adverse effect on our business, financial condition, results of operations and cash flows.
Our success depends on our customers’ confidence that we can provide reliable, consistently high-quality products, which also requires us to provide validated data to support our customers’ use of our products. We believe that customers are likely to be sensitive to our products failing to meet the specifications shown on our data sheets. Our reputation and the public perception of our products and technologies may be impaired if our products fail to perform as expected or fail to meet applicable quality criteria, specifications or performance standards. If our products experience, or are perceived to experience, a material defect or error, this could result in loss or delay of sales, damaged reputation, diversion of development resources and increased insurance or warranty costs, any of which could harm our business. These risks may be amplified by our new product lines as we implement appropriate quality control criteria. We are reliant, to an extent, on customer feedback on the quality of our products, and it may take additional time for new products to meet the desired quality standards. Any defects or errors could also result in our inability to timely deliver products to our customers, which could cause disruptions to our customers’ ability to obtain results, narrowing the scope of the use of our products and ultimately hindering our or their success in relevant markets. Even after any underlying concerns or problems are resolved, any lingering concerns regarding our technology, product defects or performance standards could continue to result in lost sales, delayed market acceptance and damaged reputation, among other things. If problems in preparation or manufacture of a product, failure to meet required quality standards for that product or other product defects are not discovered before such product is released to our customers, we may be subject to adverse legal or regulatory actions, including halting of manufacturing and distribution, restrictions on our operations, civil sanctions (including monetary sanctions), and criminal actions. In addition, such problems or failures subject us to other litigation claims, including claims from our customers for reimbursement of the cost of lost or damaged materials. Our customers also require specific information regarding our products and their uses, and any inaccuracies in this information could lead to products being sold for the wrong uses and may result in
4
our having to refund or replace the products in question. Any of the above problems may adversely affect our reputation, business, financial condition and results of operations.
We must develop new products and enhance existing products, adapt to significant technological and innovative changes and respond to introductions of new products by competitors in order to remain competitive.
We sell our products in industries that are characterized by significant technological changes, frequent new product and technology introductions and enhancements and evolving regulatory requirements and industry standards. As a result, our customers’ needs continue to evolve and our products may be superseded by new technologies (for instance, if certain drugs are no longer administered through injection) or their demand may decline. For instance, as our sales and profitability are largely dependent on the sale of products delivered by injection, if our customers reconfigure their drug product or develop new drug products requiring less frequent dosing, our sales and profitability may suffer. Likewise, if we do not appropriately innovate and invest in new products and technologies, and be open to broadening the scope of our offerings, our product offerings may become less desirable in the markets we serve, and, although changing providers is a lengthy process for our customers, they could move to new technologies offered by our competitors, especially if such competitors are able to react more directly and effectively to a customer’s specific demand. Though we believe customers in our markets display a significant amount of loyalty to a particular product, we also believe that because of the initial time investment required by many of our customers to reach a purchasing decision for a new product, it may be difficult to regain a customer once that customer purchases a product from a competitor.
Moreover, there is a risk that significant amounts of time and resources that we invest in research, development and identification of new products may not result in the expected positive results for our business. If we invest our resources into a new product or product enhancements that fail to meet our high-quality standards and market expectations or do not perform as intended, this could adversely affect our business. Our current customers may decide not to purchase these new products or product enhancements and / or purchase a product from a competitor or cease doing business with us altogether. It can take significant time to identify an unmet customer need and develop a product to meet that need, and to the extent we fail to obtain desired levels of market acceptance, our business, financial condition or results of operations could be adversely affected.
Our estimates of our addressable market include several key assumptions based on our industry knowledge, industry publications, third-party research and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, no independent source has verified such assumptions. Industry publications, research, surveys, studies and forecasts generally state that the information they contain has been obtained from sources believed to be reliable.
If any of our assumptions or estimates, or these publications, research, surveys or studies prove to be inaccurate, then the actual market for our products may be smaller than we expect, and as a result, our product revenue may be limited and our business, financial condition or results of operations could be adversely affected.
Our backlog might not accurately predict our future revenue, and we might not realize all or any part of the anticipated revenue reflected in our backlog.
Our backlog represents, as of a point in time, estimated future revenue for work not yet completed under (i) specific purchase orders, with regards to our Biopharmaceutical and Diagnostic Solution segment, and (ii) certain one-off agreements, with regards to our Engineering segment, where we typically recognize direct revenue over the life of the contract based on our performance of services under the contract. Contracts may be terminated or delayed by our customers or regulatory authorities for reasons beyond our control. To the extent projects are delayed, the timing of our revenue could be affected. In the event a customer terminates a contract, we are generally entitled to be paid for services rendered through the termination date and for services provided in winding down the project. However, we are generally not entitled to receive the full amount of direct revenue reflected in our backlog in the event of a contract termination. The duration of the projects in our backlog, and the related revenue recognition, generally ranges from several months to many years. For orders that are placed inside a contractual firm period, we generally have a contractual right to payment in the event of cancellation. Fluctuations in our reported backlog levels also result from the timing and order pattern of our customers who often seek to manage their level of inventory on hand. Because of customer ordering patterns, our backlog reported for certain periods may fluctuate and may not be indicative of future results. A number of factors may affect backlog and the direct revenue generated from our backlog, including:
5
Our backlog at December 31, 2023 was approximately €944.9 million compared to €957.0 million as of December 31, 2022. Although an increase in backlog will generally result in an increase in future direct revenue to be recognized over time (depending on future contract modifications, contract cancellations and other adjustments), an increase in backlog at a particular point in time does not necessarily correspond to an increase in direct revenues during a particular period. The timing and extent to which backlog will result in direct revenue depends on many factors, including the production and timing expected by our customers, the timing of the commencement of work, the rate at which we perform services, scope changes, cancellations, delays, receipt of regulatory approvals and the nature, duration, size, complexity and phase of the project. In addition, the uncompleted portion of delayed projects remain in backlog until they are canceled or completed. As a result of these factors, our backlog is not necessarily a reliable indicator of future direct revenue and we might not realize all or any part of the direct revenue from the authorizations in backlog as of any point in time.
If we fail to maintain and enhance our brand and reputation, our business, results of operations and prospects may be materially and adversely affected.
We believe that maintaining and enhancing our brand and reputation are of significant importance to the success of our business. We work to set a very high standard for the quality of our products and our ethical business practices, and we believe that this has been crucial to our success. We have employed and will continue to employ different types of consumer experience and interaction engagements designed to gauge consumer satisfaction with our products, and we also engage in rigorous product validation in order to continue to improve our product quality. We cannot assure you, however, that these activities will be successful or that we will be able to continue to maintain our brand and reputation as we expect. If our brand strength deteriorates, or if our brand is no longer associated with high-quality products, it could lead to fewer publication citations for our products, which could in turn further weaken our brand recognition and reputation. In addition, our competitors may increase the intensity of their consumer interactions or customer feedback processes, which may force us to increase our advertising spend to engage with our customer base and maintain brand and reputational awareness.
In addition, any negative publicity relating to our products or services, regardless of its veracity, could harm our brand and the perception of our brand in the market. With an increasing global focus on ethical business practices and good corporate behavior, and with such issues directly influencing consumer behavior, any failure to achieve or maintain the levels of corporate governance, social and environmental impact and corporate behaviors expected of us, including demonstrating dedication to the benefits of diversity, could negatively impact our brand and reputation.
If our brand is harmed, we may not be able to gain new customers or continue to maintain positive relationships with our customers, and our business, prospects, financial condition and results of operations could be materially and adversely affected.
Part of our growth strategy is to increase direct customer interactions in multiple countries. Failure to anticipate and react to particular geographic requirements and sensitivities may have a negative impact on our brand and reputation, which may result in a decrease in sales or sales growth in such countries, which may adversely affect our business, prospects, financial condition and results of operations.
We are highly dependent on our management and employees. Competition for our employees is intense, and we may not be able to attract and retain the highly skilled employees that we need to support our business and our intended future growth.
Our success largely depends on the skills, experience and continued efforts of our management, including our Executive Chairman, our Chief Executive Officer and our senior leadership, as well as of our research and development and highly skilled employees. The replacement of certain members of our global leadership team would likely involve the expenditure of significant time and financial resources, and the loss of any such individual may significantly delay or prevent the achievement of our business objectives. Likewise, the members of our research and development team and our highly skilled employees, whom our customers and competitors often seek to engage, may be difficult to replace in light of their sophisticated skills and experience and a shortage of such employees could
6
disrupt our operations. As we continue to grow, our success also depends on our ability to attract, motivate and retain highly qualified individuals who will also fit within our culture. Competition for senior management and other personnel in our industry is intense, and the pool of suitable candidates is limited. If qualified personnel become scarce or difficult to attract or retain in our industry for compensation-related or other reasons, we could experience higher labor, recruiting or training costs. Further, new hires may require significant training and time before they achieve full productivity and may not become as productive as we expect. The failure to attract, retain and properly motivate members of our senior management team and other employees, to find suitable replacements for them in the event of death, illness or their desire to pursue other professional opportunities, or to maintain our corporate culture as we continue to grow, could have a negative effect on our operating results.
Our business, financial condition and results of operations depend upon maintaining our relationships with suppliers and service providers.
Our results of operations have been, and will continue to be, dependent in part on our ability to obtain favorable terms from our suppliers and services providers, including logistics service providers. These terms may change from time to time, and such changes could adversely affect our gross margins over time. In addition, our cash flows could be adversely impacted by the acceleration of payment terms to our suppliers and/or the imposition of more restrictive credit terms and other contractual requirements. Further, if for any reason we enter into a contract with a supplier on unfavorable terms, it may harm our ability to negotiate our future contracts with that supplier or with other suppliers.
The loss of one or more of our large suppliers including as a result of consolidation, a material reduction in their supply of products or provision of services to us, extended disruptions or interruptions in their operations or material changes in the terms we obtain from them, could have a material adverse effect on our business, financial condition and results of operations.
Our business, financial condition and results of operations depend upon the availability and price of high-quality materials and energy supplies and our ability to contain production costs.
Our operations depend upon our ability to obtain high-quality materials and energy supplies at reasonable prices, therefore maintaining low production costs. Our ability to maintain an adequate supply of such materials and energy could be impacted by the availability and price of those materials and energy, the failure to maintain relationships with suppliers and any of such materials being proven to be toxic or otherwise inadequate to be used for the intended purpose. While we may seek to minimize the impact of price increases and potential shortages by, among other things, entering into long-term supply agreements, maintaining commercial relationships with multiple suppliers when possible, increasing our own prices and implementing cost-saving measures, our result of operations and cash flows could be adversely affected in the event these measures are insufficient to cover our costs. With respect to energy supplies, the current conflict between Russia and Ukraine and the financial and economic sanctions imposed by the European Union, the U.S., the United Kingdom and other countries and organizations against officials, individuals, regions, and industries in Russia and Belarus have caused significant fluctuations in gas and energy prices, and may further negatively impact our ability to source gas at commercially reasonable terms, or at all. Moreover, while we aim to maintain a large network of product suppliers, we are unable to predict any interruption or disruption in service from our key suppliers. In particular, for some of the materials we use in our production cycles we have a limited number of (or a single-source) suppliers worldwide, and selecting new suppliers would be a lengthy and time consuming process. Any interruption or disruption in service from particular suppliers of materials could lead to interruptions or stoppages in such deliveries which could, in turn, adversely affect our operations until arrangements with alternative suppliers are put into place. If this occurs, we could expend substantial resources and time in re-establishing relationships with third-party suppliers that meet the appropriate quality, cost and regulatory requirements needed for commercially viable manufacturing of our products. If we are unable to obtain the materials we need at reasonable prices or at all, we may not be able to produce certain of our products at a marketable price or at all. If our supply of materials and components is adversely affected, including as a result of the conflict between Russia and Ukraine and the conflicts in the Middle East, we could damage our relationship with current and prospective customers and our operating results and financial condition could be adversely affected.
Moreover, we are dependent upon the ability of our suppliers to provide materials that meet our quality standards, as well as delivery schedules. Our suppliers’ failure to provide expected materials that meet such criteria could adversely affect production schedules and contract profitability.
7
The continued supply of high-quality third-party materials and energy from our suppliers is subject to a number of risks, including:
Moreover, global cost inflation trends have had an effect on and could continue to unfavorably impact pricing from our suppliers, which in turn could impact our gross margins to the extent we are unable to pass along price differences to our customers.
If we experience problems with suppliers, we may not be able to find acceptable alternatives, and any such alternatives could result in increased costs for us and possible forward losses on certain contracts. Even if acceptable alternatives are found, the process of locating and securing such alternatives might be disruptive to our business, might lead to termination of our supply agreements with our customers and might disrupt the operations of our customers leading to potential claims, any of which could adversely affect our business, financial condition and results of operations.
Significant interruptions in our operations could harm our business, financial condition and results of operations.
Manufacturing, distribution, service and logistics problems can and do arise, particularly in light of geopolitical conflicts, and any such problems could have a significant impact on our business, financial condition and results of operations. Accordingly, any significant disruptions to the operations of our manufacturing or distribution centers or logistics providers for any reason, including labor relations issues, power interruptions, severe weather, fire or other circumstances beyond our control could cause our cost of sales and operating expenses to increase without coverage or compensation or seriously harm our ability to fulfill our customers’ orders or deliver products on a timely basis, or both. Likewise, our ability to meet our customers’ needs and expectations may be frustrated by delays, issues or interruptions in ramping up new production lines or plants. We must also maintain sufficient production capacity in order to meet anticipated customer demand, which carries fixed costs that we may not be able to offset if orders slow, which would adversely affect our operating margins. If we are unable to manufacture our products consistently, in sufficient quantities and on a timely basis, our revenue, gross margins and our other operating results will be materially and adversely affected. Prompt shipment of our products is also very important to our business. If we experience significant delays in our manufacturing, shipping or logistics processes, this could cause disruption to our customers and damage our current and future customer relationships and may adversely affect our business. Such delays may also adversely impact our new product development. For example, if we were to lose one of our sites where new product development is undertaken, we may not be able to transfer or replicate that product development at another site, with the result of lost time and financial costs of developing the new product. We may also use high-risk chemicals in the manufacture of certain of our products, which are subject to handling risks, and any disruption in our ability to source or appropriately store these chemicals could adversely affect our manufacturing operations.
8
As a consequence of the COVID-19 pandemic, sales of syringes and vials to and for vaccination programs had globally increased resulting in a revenue growth acceleration. The demand for such products related to COVID-19 may continue to shrink, if the need for COVID-19 related solutions continues to decline.
During COVID-19, we increased production capacity to support our customers’ efforts in rapidly responding to COVID-19. In this context we have provided: (i) glass vials and syringes to approximately 90% of marketed vaccine programs, according to our estimates based on public information (WHO, EMA, FDA); and (ii) plastic diagnostic consumables for the detection and diagnosis of COVID-19. COVID-19 generated increased demand for our products and services. We expect demand for COVID-19 related products to continue to reduce as a result of the transition from pandemic to endemic status.
There remains some uncertainty around the magnitude of the long-term impact of COVID-19 on demand for our products, but we anticipate that COVID-19 contributions will continue to decrease and potentially transition into a part of our traditional vaccination business.
A lower rate of increase or a decline in sales of syringes and vials to and for vaccination programs, plastic diagnostic consumables for COVID-19 testing, and related products and services could adversely affect our business, financial condition and results of operations.
Our manufacturing facilities are subject to operating hazards which may lead to production curtailments or shutdowns and have an adverse effect on our business, results of operations, financial condition or cash flows.
Certain of our manufacturing processes involve heating glass to extremely high temperatures, forming plastic and operating heavy machinery and equipment, which entail a number of risks and hazards, including industrial accidents, leaks and ruptures, explosions, fires, mechanical failures and environmental hazards, such as spills, storage tank leaks, discharges or releases of toxic or hazardous substances and gases, including into the environment. Any of these events, which are generally more likely to occur as our machines approach time for refurbishment, could lead to requirements for environmental remediation and civil, criminal and administrative sanctions and liabilities. These hazards may cause unplanned business interruptions (also as a consequence of remediation actions), unscheduled downtime, transportation interruptions, personal injury and loss of life, severe damage to or the destruction of property and equipment, environmental contamination and other environmental damage, civil, criminal and administrative sanctions and liabilities and third-party claims, any of which may have a material adverse effect on our business, financial condition, results of operations and cash flows.
In addition, under applicable local laws, including Italian law, our directors and officers may be subject to criminal liability, in connection with injuries occurred to our employees, as a result of workplace health and safety violations by reason of their position as employers (posizione di garanzia). Convictions of our directors and officers could negatively impact our reputation. Moreover, due to the long industrial history of our manufacturing facilities and the subsequent lack of detailed information regarding historical waste and chemical storage and disposal, the risk of soil, water or groundwater contamination and related civil, administrative and criminal liabilities cannot be eliminated.
We may face significant competition in implementing our strategies for revenue growth in light of actions taken by our competitors.
In each business segment in which we operate, we face significant competition, with many competitors focusing on specific regions, customers and/or specific product segments. Competitors range from smaller, specialized companies, which may be able to more quickly respond to customers’ specific needs, to large multinational companies who provide a full suite of products, which may have greater financial, marketing, operational and research and development resources than we do. Such greater resources may allow our competitors to respond more effectively with new, alternative or emerging technologies. Failure to anticipate and respond to our competitors’ actions may impact our future sales and earnings, in particular failure to react to competitors strengthening their brand, marketing or customer experience may negatively impact our ability to attract and retain customers.
We are pursuing a number of strategies to maintain and improve our revenue growth, including:
9
We may not be able to successfully implement these strategies, and these strategies may not result in the desired growth of our business. Failure to anticipate and respond to our competitors’ actions may adversely affect our business, financial condition and results of operations.
Our business may be harmed if our customers discontinue or spend less on research, development, production or other scientific endeavors.
Our customer base includes leading pharmaceutical, biologic, diagnostic and medical device companies worldwide. Many factors, including public policy spending priorities, available resources and product and economic cycles, have a significant effect on the capital spending policies of these entities. For instance, any change in the international healthcare systems, including the Patient Protection and Affordable Care Act (the “PPACA”) in the U.S., resulting in a reduced ability of pharmaceutical companies and healthcare providers to receive reimbursements by government authorities, private insurers and other third-party payers for the costs of our products, could result in reduced demand for our products.
More recently, the Inflation Reduction Act (the "IRA") was signed into law. Among other things, the IRA requires manufacturers of certain drugs to engage in price negotiations with Medicare (beginning in 2026) with prices that can be negotiated subject to a cap; imposed rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation (first due in 2023); and replaces the Part D coverage gap discount program with a new discounting program (beginning in 2025), all factors which could impact our business by affecting our ability to achieve value-based price, maintaining an acceptable return on our investments in R&D of our products, creating a potential financial impact to the Company to the extent our products are used in connection with drugs that are impacted by the IRA pricing provisions under the IRA and impacting our ability to research and develop new products.
Fluctuations in the research and development budgets of our customers could have a significant effect on the demand for our products. Our customers determine their research and development budgets based on several factors, including the need to develop new products, continued availability of governmental funding and other incentives, competition and the general availability of resources. Any reduction in research and development budgets or a shift of any funding source currently allocated to our business sector to different areas of research, could adversely affect our business, financial condition and results of operations.
The loss of a significant number of customers or a reduction in orders from a significant number of customers could reduce our sales and harm our financial performance.
Our operating results could be negatively affected by the loss of revenue from a significant number of our customers. Our revenue is fairly well distributed, with 45.5% of our revenues deriving from our top ten customers and one individual customer representing 11.4% of revenues in 2023. However, consolidation within our customer base,
10
including, in particular, among pharmaceutical companies, may give larger customers greater bargaining and buying power and operational sophistication, which can enable them to operate with reduced inventories. In addition, consolidation among our customers may lead them to rely on a reduced number of suppliers, with no assurance that they will continue using our products.
We maintain close business relationships with certain customers, working closely to build the specific custom tools they need, which will then become part of our product portfolio. Our operating results could be adversely affected by the loss of a significant number of these customers, particularly during the product development phase.
Our customer contracts generally do not contain minimum purchase requirements, and a significant portion of our sales are on a purchase order basis. Therefore, our customers are generally not obligated to purchase any fixed quantities of products, and they may stop placing orders with us at any time. If a significant number of customers purchase fewer of our products, defer orders or fail to place additional orders with us for any reason, our sales could decline, and our operating results may not meet our expectations. In addition, if those customers order our products, but fail to pay on time or at all, our liquidity and operating results could be adversely affected.
The level and timing of orders placed by our customers vary for different reasons, including individual customer strategies, the introduction of new technologies, the desire of our customers to reduce their exposure to any single supplier and general economic conditions. If for example customers vary or decrease the level of inventories they are holding of our products at their sites by engaging in de-stocking or new ordering patterns, or if we otherwise have decreased visibility of our products held by or on behalf of our customers, our business could be adversely affected.
If we are unable to anticipate and respond to the demands of our customers, if we have an inadequate supply of products, insufficient capacity in our sites or if we experience any disruptions to our supply chain or distribution network, we may lose customers. Alternatively, we may have excess inventory or excess capacity, and either of these factors may have a material adverse effect on our business, financial condition and results of operations.
Our business may suffer if we do not successfully manage our current and potential future growth.
Over the last 70 years we have consistently expanded our operations and anticipate expanding further as we pursue our long-term growth strategy. The key elements of our growth strategy include, among other things, the expansion of our global market position in drug containment solutions and drug delivery systems, accelerating penetration in life sciences systems, increasing our investments in research and development, building on our expertise in manufacturing, assembly and inspection systems for drug containers and complex, multi-component systems, leveraging our scientific and engineering capabilities, increasing our penetration in the North American and APAC regions and selectively pursuing acquisitions and technology partnerships to augment and expand our product and service portfolio. We have further expanded our manufacturing facilities in Piombino Dese (Italy) and established new plants primarily for EZ-Fill® products, with strong focus on biologics and vaccines, in Fishers (Indiana, U.S.), and Latina (Italy) and evaluated strategic acquisitions to broaden our offering, our technical know-how and our international footprint. In November 2021, we entered into an investment agreement with the Zhangjiagang Economic and Technological Development Zone Administration Committee. Under said investment agreement, we agreed to invest through one of our subsidiaries, in the Zhangjiagang Economic and Technological Development Zone ("ZETDZ"), to be used for the manufacture of ready to use syringes and vials, bulk vials and cartridges, and for the SG Engineering business. In March 2023, we we decided to delay the timing of our investment in China in order to focus on the execution of our new manufacturing facilities in the United States and Italy.
In December 2021, we entered into an Early Development Agreement (“EDA”) with the City of Fishers – Indiana, Fishers Town Hall Building Corporation and City of Fishers Redevelopment Commission envisaging the acquisition of an area of approximately 35.75 acres to be used for the construction of a new plant in Indiana (U.S.). The new manufacturing plant is expected to launch commercial production in 2024.
In addition, on May 27, 2022, Nuova Ompi acquired a brownfield in Latina (Italy) in proximity to other Stevanato Group facilities for a total consideration of approximately €16.0 million. The facility, after renovation, is expected to produce EZ-fill® syringes and cartridges. The new manufacturing plant launched commercial production in the fourth quarter of 2023.
11
On November 8, 2023, we acquired all of the business operations of Perugini S.r.l., an Italian company specialized in the manufacturing of consumables and mechanical components for industrial machines for a total consideration of approximately €6.4 million. The acquisition of Perugini will support the Group's efforts in the ongoing integration of critical technologies and processes into the Group’s production process.
Establishing new production plants for EZ-Fill® products represents a priority in light of the risks associated with our Piombino Dese (Italy) manufacturing facilities currently being the only ones devoted to the production of EZ-Fill® products which, in turn, exposes our business to risks of material disruption should any event affect the operation of such facilities. In general, such growth strategy and in particular the facilities expansion and the external acquisitions increase the complexity of our business and place a significant strain on our management, operations, technical systems, financial resources and internal control over financial reporting functions. Our current and planned personnel, systems, procedures and controls may not be adequate to support and effectively manage our future operations, especially as we employ personnel and maintain manufacturing facilities and distribution networks in several geographic locations.
We are also continuously expanding our product portfolio, and establishing and developing new products require significant management time and attention. If these products do not achieve the anticipated success or require greater levels of time and investment to reach the expected levels, it could adversely affect our business, financial condition and results of operations. Failure to appropriately integrate new products and business lines into our existing operations and systems can also affect the success of these products, and failure to adequately anticipate and plan for this integration could affect the success of these products and may also negatively impact our existing product offerings.
We may not successfully identify or integrate acquired businesses or assets into our operations or be able to fully recognize the anticipated benefits of businesses or assets that we acquire.
We consider acquisitions a useful instrument to complement our organic growth. We opportunistically explore acquiring other businesses and assets, and we have several acquisitions over the last few years, including: the acquisition of a 65% stake in the Danish SVM Automatik in February 2016 and of the remaining 35% in October 2021, the acquisition of the operating unit of Balda Group in March 2016, the acquisition of Medirio in May 2016 and the acquisition of the remaining 49% of our subsidiary Ompi of Japan Co. Ltd in July 2023.
On November 8, 2023, we acquired all of the business operations of Perugini S.r.l., an Italian company specialized in the manufacturing of consumables and mechanical components for industrial machines for a total consideration of approximately €6.4 million. The acquisition of Perugini will support the Group's efforts in the ongoing integration of critical technologies and processes into the Group’s production process.
However, we may be unable to identify or complete promising acquisitions for many reasons, including any misjudgment of the key elements of an acquisition, competition among buyers, the high valuations of businesses in our industry, the need for regulatory and other approvals, lack of internal resources to successfully pursue all attractive opportunities and availability of capital.
When we do identify and complete acquisitions, we may face financial, managerial and operational challenges, including diversion of management attention and resources needed for existing operations, difficulties with integrating acquired businesses, integration of different corporate cultures, increased expenses, potential dilution of our brand, assumption of unknown liabilities, potential disputes with the sellers and the need to evaluate the financial systems of and establish internal controls for acquired entities. Further, we seek out acquisitions of companies that maintain the same high quality standards that we maintain, and if we misjudge or overestimate a company’s product quality standards, we may not be able to use these products or implement the strategies that were the primary reason for the acquisition, which would lead to a significant loss both financially and in time spent by our teams trying to integrate the product or implement the strategy. There can be no assurance that we will engage in any additional acquisitions or that we will be able to do so on terms that will result in any expected benefits.
In addition, our ability to realize the benefits we anticipate from our acquisition activities, including any anticipated sales growth, cost synergies and other anticipated benefits, will depend in large part upon whether we are able to integrate such businesses efficiently and effectively. Integration is an ongoing process, and we may not be able to fully integrate such businesses smoothly or successfully, and the process may take longer than expected. Further, the integration of certain operations and the differences in operational culture following such activity will continue to
12
require the dedication of significant management resources, which may distract management’s attention from day-to-day business operations.
There may also be unasserted claims or assessments that we failed or were unable to discover or identify in the course of performing due diligence investigations of target businesses. While we normally negotiate representation and warranties and related indemnification in relation to such acquisitions, these may not be enough to cover our exposure if a significant liability arises in connection with any acquisition agreement. We cannot assure you that these indemnification provisions will protect us fully or at all, and as a result we may face unexpected liabilities that could adversely affect our business, financial condition and results of operations.
If we are unable to successfully integrate the operations of acquired businesses into our business, we may be unable to realize the sales growth, cost synergies and other anticipated benefits of such transactions, and our business, results of operations and cash flow could be adversely affected.
Our reputation, ability to do business and results of operations may be impaired by improper conduct by any of our employees, agents or business partners.
We cannot provide assurance that our internal controls and compliance systems will always protect us from acts committed by employees, agents or business partners of ours (including third-party suppliers, distributors or of businesses we acquire or partner with) that would violate U.S. and/or other national laws, including the laws governing payments to government officials, bribery, fraud, kickbacks and false claims, pricing, sales and marketing practices, conflicts of interest, competition, export and import compliance, money laundering and data privacy. In particular, the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act and similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from making improper payments for the purpose of obtaining or retaining business. Any improper actions by our employees, suppliers and distributors or allegations of such acts could damage our reputation and subject us to civil or criminal investigations in Italy, under Italian Legislative Decree No. 231 of June 8, 2001 (the “Decree 231”) pursuant to which a legal entity can be held liable to pay fines in connection with certain criminal offenses committed, inter alia, by its directors, officers or employees, the United States and in other jurisdictions, and any related shareholder lawsuits could lead to substantial civil and criminal, monetary and nonmonetary penalties and could cause us to incur significant legal and investigatory fees. In particular, pursuant to Decree 231, a defense can be established by an entity involved in a Decree 231 investigation, if such entity can prove, among others, that it adopted and properly implemented an organization, management and control model aimed at effectively preventing the commission of the criminal acts involved prior to such unlawful conduct having taken place. We approved and adopted the current (fourth) version of our organization, management and control model provided by Decree 231 (“Model 231”) by means of a resolution of the board of directors dated November 4, 2022, and appointed the current supervisory body (the “Supervisory Body”) that supervises the functioning of and compliance with Model 231, and monitors and assesses the implementation status of preventive measures, with regular yearly reports to the board of directors. The adoption of organization and management models does not by itself exclude applicability of the penalties provided by Decree 231. In fact, upon commission of an offense resulting in administrative liability of the Company pursuant to Decree 231, the court will evaluate the models and their actual implementation.
Failure to comply with Decree 231 could result in the imposition of administrative sanctions such as monetary sanctions and other types of sanctions, if applicable (e.g., interdictory sanctions, including prohibitions such as participation in public tenders or the termination of a public contract already awarded, confiscation of the price or profits deriving from the crime and publication of the judgment) and loss of confidence of our customer base, which could have a material adverse effect on the business, financial condition, results of operations and prospects of the Group.
In addition, a government may seek to hold us liable as a successor for violations committed by companies in which we invest or that we acquire.
We also rely on our suppliers to adhere to our supplier standards of conduct, and material violations of such standards of conduct could occur that could have a material effect on our business, reputation, financial condition and results of operations.
13
Our global operations are subject to international market risks that may have a material effect on our liquidity, financial condition, results of operations and cash flows.
We operate manufacturing facilities in Italy, Slovakia, Denmark, Germany, United States, Mexico, China and Brazil, and sell and distribute our products in more than 70 countries. As part of our business strategy, we will continue to seek to expand our sales and market share in various international markets in which we currently operate and evaluate expansion opportunities into additional international markets. The economies of some of these markets differ from the economies of our core markets factors in Europe and in some cases present new and greater risks. Our financial results and operations are substantially dependent upon macro-economic and political conditions, particularly in Italy, Slovakia, Denmark, Germany, United States, Mexico, China and Brazil, where we operate manufacturing facilities. High levels of sovereign debt in certain countries (including Italy), combined with increasing inflation, weak growth, political instability and high unemployment rates, could lead to additional fiscal reforms (including austerity measures), sovereign debt restructurings, currency instability, increased counterparty credit risk, high levels of volatility and, potentially, disruptions in the credit and equity markets, as well as other outcomes, each of which, alone or combined with other factors, could have a material adverse effect on our business, results of operations, access to credit and capital markets and, therefore, our ability to implement our growth strategy.
Global conflicts, including the recent developments in the Middle East and the ongoing military actions undertaken by Russian military forces against Ukraine have created and are likely to continue creating substantial disruptions. In particular, military actions against Ukraine, as well as the measures adopted, or that may be adopted, by other countries in response to these events, including new and stricter sanctions by the European Union, the U.S., the United Kingdom and other countries and organizations against officials, individuals, regions, and industries in Russia and Belarus (or other countries that were to become involved), have unfavorably affected our operations primarily due to fluctuations in gas and electricity prices, and could have a material adverse effect on our operations going forward. We are monitoring the conflict and continue to attempt to mitigate the effects of such conflict on our operations to the extent possible, but do not and cannot know if this situation may result in additional broader economic and security conditions or in material implications for our business. Currently, the Group's operations in Italy have the highest gas consumption across its European operations. In 2022 and continuing in 2023 the Italian government has taken steps to shore up its natural gas supplies and lower its dependence on Russian supplies. Italy has signed agreements with several other countries to diversify the country's natural gas sources, and Algeria is now the largest supplier of natural gas to Italy. In addition, the Group believes it may be eligible for priority status since its business operations are devoted to the delivery of mission-critical pharmaceutical products.
Macro-economic difficulties and political instability remain particularly evident in Italy. The Italian economy, along with certain other European economies, has from time to time experienced significant financial market volatility and economic adversity due to concerns about economic downturn, political instability and rising government debt levels. Interest rates on Italy's sovereign debt may rise to levels that may make it difficult for it to service high debt levels without significant financial help from the EU and could potentially lead to default. These events have in the past adversely impacted the Italian economy, causing credit agencies to lower Italy’s sovereign debt rating, and could decrease outside investment in Italian companies. Any further downgrade of the Italian sovereign debt rating could create additional economic uncertainty and negatively impact Italy’s growth, which could in turn affect consumer confidence, discretionary spending and, consequently, demand for our products.
Furthermore, policies, measures, controls or other actions implemented by the governments of emerging markets or countries which we target for increased sales may restrict our business operations or harm our financial results.
As a result, our revenue is exposed to risks inherent to the country where we operate or intend to operate including risks related to differing political, legal, regulatory and economic conditions and regulations.
As a multinational corporation, we are exposed to fluctuations in currency exchange rates and interest rates, which could adversely affect our cash flows and results of operations.
International markets contribute a substantial portion of our revenue, and we intend to continue expanding our presence globally. The exposure to fluctuations in currency exchange rates takes on different forms. Revenue and costs are subject to the risk that fluctuations in exchange rates could adversely affect our reported revenue and profitability when translated into Euro for financial reporting purposes. These fluctuations could also adversely affect the demand for products and services provided by us. As a multinational corporation, our businesses often invoice
14
third-party customers in currencies other than the one in which they primarily do business (the “functional currency”), especially U.S. Dollars and the Mexican Pesos. Movements in the invoiced currency relative to the functional currency could adversely impact our cash flows and our results of operations. As our international sales grow, exposure to fluctuations in currency exchange rates could have a larger effect on our financial results. Similarly, the current conflict between Russia and Ukraine has created extreme volatility in the capital markets and is expected to have further global economic consequences.
The deterioration of the sovereign debt of several countries, together with the risk of contagion to other, more stable, countries, has exacerbated the global economic crisis. In particular, a deterioration in general economic conditions caused by instability in the Eurozone could have a material adverse effect on our business, financial condition, results of operations and prospects.
We are required to comply with a wide variety of laws and regulations and are subject to regulation by various federal, state and foreign agencies.
In all the jurisdictions in which we operate, we are subject to a number of laws, regulations and practices concerning, inter alia, the health and safety of our employees, the use, manufacture and importing of chemicals and the protection of the environment and natural resources.
In the event that the applicable laws and regulations were to change such that our products or our production processes were subject to greater regulatory control or restrictions, it could have a significant impact on our ability to market and sell our products and could require us to spend significant amounts to ensure and monitor compliance with such laws and regulations such that our business, financial condition and results of operations could be adversely affected. For instance, both the EU and the United States are considering to further restrict in the next years the use of ethylene oxide, the main sterilizing agent used in our production processes. If the use of ethylene oxide is further restricted, or completely banned, this would require us to identify new sterilizing agents and would have a negative impact on our financial condition and results of operations.
We are also subject to a variety of federal, state, local and international laws and regulations that govern, among other things, the importation and exportation of products, the handling, transportation and manufacture of substances that could be classified as hazardous, laws governing government contracts and our business practices such as anti-corruption and antitrust laws. Although we believe that we comply in all material respects with applicable laws and regulations, there can be no assurance that a regulatory agency or tribunal would not reach a different conclusion concerning the compliance of our operations with applicable laws and regulations.
In addition, there can be no assurance that we will be able to maintain or renew existing permits, licenses or other regulatory approvals or obtain, without significant delay, future permits, licenses or other approvals needed for the operation of our businesses. Furthermore, loss of a permit, license or other approval in any one portion of our business may have indirect consequences in other portions of our business if regulators or customers, for example, cease doing business with such other portion due to fears that such loss is a sign of broader concerns about our ability to deliver products or services of sufficient quality.
Any noncompliance by us with applicable laws and regulations or the failure to maintain, renew or obtain necessary permits and licenses could have an adverse effect on our business, financial condition and results of operations.
Failure to comply with these laws and regulations can lead to agency action, including warning letters, product recalls, product seizures, monetary sanctions, injunctions to halt manufacturing or distribution, restrictions on our operations, withdrawal of existing or denial of future approvals, permits or registrations, including those relating to products or facilities and civil and criminal sanctions. To the extent these agencies were to take enforcement action, such action may be made publicly available, and such publicity could harm our ability to sell these regulated products globally and may harm our reputation. In addition, such actions could limit the ability of our customers to obtain regulatory clearance or approval for their products in the United States or abroad and/or our customers may incur significant costs in obtaining or maintaining such regulatory clearances or approvals in the United States or abroad. In addition, any such failure relating to the products we provide exposes us to direct and third-party product liability claims as well as contractual claims from our customers, including claims for reimbursement for lost or damaged products, as well as potential recall liability, which could be significant. Customers may also claim loss of profits due
15
to lost or delayed sales, although our contractual arrangements typically place limits on such claims. There can be no assurance that any such contractual limitation will be applicable or sufficient or fully enforced in any given situation.
Given the relevance of our activities in the healthcare sector, investments by non-Italian entities in the Company, as well as certain asset disposals by the Company, may be subject to the prior authorization of the Italian Government (so called “golden powers”).
Due to the relevance of our activities in the healthcare sector, it is not possible to exclude the recurrence of the conditions for the exercise, by the Italian Government, of the so called “golden powers”, aimed at impacting the liquidity and value of the Shares. The golden power regime, set forth, among others, in (i) Law Decree no. 21 of March 15, 2012 (converted into law by Law no. 56 of May 11, 2012), as amended and supplemented (“Golden Power Decree”), and (ii) Decree of the President of the Council of Ministers no. 179 of December 18, 2020, also cover the healthcare sector. With respect to such sector, the powers set forth in the Golden Power Decree include, inter alia, the powers to: (i) veto, or impose specific conditions on, the purchase by EU companies of controlling stakes or by non-EU companies of shareholdings at least equal to 10% of voting rights or corporate capital in companies having assets and relationships in sectors which are considered strategic (the “Strategic Companies”), and (ii) veto, or impose specific conditions on, the adoption of certain corporate resolutions, acts or transactions by the same companies which may pose a threat to national security.
With respect to the healthcare sector, the Golden Power Decree requires companies to notify the office of the Italian Prime Minister within 10 days of: (i) any purchase by an EU entity of a stake in a Strategic Company resulting in the buyer acquiring control of such company pursuant to article 2359 of the Italian Civil Code and of the Italian Financial Act (TUF); (ii) any purchase by a non-EU entity of either a controlling stake or a minority stake at least equal to 10% of voting rights or corporate capital in a Strategic Company; and (iii) any resolution, act or transaction adopted by a Strategic Company resulting in a transfer of ownership, control or availability of strategic assets to either an EU or a non-EU entity. The same applies to any resolution, act or transaction adopted by a Strategic Company, whose effect is to change the destination of a strategic asset, or a change in the corporate purpose of the Strategic Company. Furthermore, the regulation requires to notify the dissolution of the company or the amendment of certain provisions of their articles of association. The office of the Italian Prime Minister must exercise its power to veto the transaction or impose conditions within 45 days from the date of notice. In the interim, all rights related to the shares other than economic rights are suspended, and any decision adopted in violation of such suspension is null and void, but once the term has expired the relevant transaction can be completed. Should the office of the Italian Prime Minister veto the transaction, the buyer must sell the acquired shares or quotas within one year.
The violation of the notification obligation or of the prescriptions eventually imposed by virtue of the exercise of special powers, unless the fact constitutes a criminal offense, is subject to a pecuniary administrative sanction up to double the value of the transaction and in any case not less than one percent of the cumulative turnover achieved by the companies involved in the last financial year for which the financial statements were approved.
As a result, our ability to pursue commercial or industrial strategic resolutions, acts or transactions that involve the acquisition of, or the subscription for, our shares by a partner (or that imply an amendment to our shareholders’ structure) may be restricted by the Italian Government’s decision to exercise its special powers with respect to our business.
Therefore, the application of the golden powers regime could have a material adverse effect on our business, results of operations, financial condition or prospects.
Furthermore, in the future, our shareholders’ ability to enter into change of control or takeover transactions may be impacted by the exercise by the Italian Government of its special powers under the golden power regime. Our shareholders may not be able to transfer their interests or such a transfer may be subject to conditions, which diminish the value of the transaction and discourage investments. This may limit our shareholders’ ability to benefit from the proceeds of certain proposed asset sales or acquisitions or business combinations, and may limit our shareholders’ ability to benefit from possible premiums connected to a proposed change of control transaction, tender offer or other strategic transactions.
16
The laws and regulations which we are subject to, such as U.S., EU and other anti-corruption laws, trade controls, economic sanctions and similar laws and regulations in the jurisdictions which we operate, are complex and the regulatory and political regimes under which we operate are volatile. Our failure to comply with the relevant laws and regulations could subject us to civil, criminal and administrative penalties and harm our reputation.
Certain of our operations are subject to U.S., EU and foreign anti-corruption and trade control laws and regulations, such as the Foreign Corrupt Practices Act (the “FCPA”), export controls and economic sanctions programs, including those administered by the U.S. Treasury Department’s Office of Foreign Assets Control (“OFAC”). As a result of doing business in foreign countries and with foreign partners, we may be exposed to a heightened risk of violating anti-corruption, export control, and sanctions laws and regulations.
The FCPA prohibits us from providing anything of value to foreign officials for the purposes of obtaining or retaining business or securing any improper business advantage. It also requires us to keep books and records that accurately and fairly reflect our transactions. As part of our business, we may deal with state-owned business enterprises, the employees of which are considered foreign officials for purposes of the FCPA. Other anti-corruption legislation which we may be required to adhere to, sets out wider prohibitions including against private bribery, which is also relevant to our business.
Economic sanctions and export controls may restrict our ability to conduct business with or in certain jurisdictions, individuals and entities. We are not a U.S. person and are not owned or controlled by one or more U.S. persons. We have in the past engaged in dealings with parties in Cuba, Iran, and Syria, and we have or could have ongoing de minimis activities with parties in Iran and Cuba. We believe that such activities have been conducted in compliance with all applicable sanctions and export controls, and are implementing policies and procedures designed to ensure continued compliance. However, we cannot be certain that these safeguards will be fully effective in the future to ensure compliance, and the scope and reach of U.S. sanctions laws could also change over time.
The military actions undertaken by Russian military forces against Ukraine in 2022 resulted in the imposition of financial and economic sanctions by the European Union, the U.S., the United Kingdom and other countries and organizations against officials, individuals, regions, and industries in Russia and Belarus. Such sanctions, together with any additional measure that may be adopted in connection with this situation, may, in various ways, constrain Russia and Ukraine related transactions. Our ability to engage in activity with certain consumer and institutional businesses in Russia and Ukraine or involving certain Russian or Ukrainian businesses and customers is dependent in part upon whether such engagement is restricted under any current or expected U.S., EU, U.K. or other countries sanctions and laws. Our ability to engage may be further impaired in the event other countries were to become involved in the conflict and, as result, be subjected to sanctions or similar restrictions.
Violations of anti-corruption, export control and sanctions laws and regulations are punishable by civil penalties, including fines, denial of export privileges, injunctions, asset seizures, debarment from government contracts and revocations or restrictions of licenses, as well as criminal fines and imprisonment. There can be no assurance that all of our employees, consultants, agents or other associated persons will not take actions in violation of these laws and regulations, and that our procedures will effectively prevent us from violating these regulations in every transaction in which we may engage or provide a defense to any alleged violation. In particular, we may be held liable for the actions that our local strategic partners take inside or outside of the United States, even though our partners may not be subject to these laws. Such a violation, even if our policies prohibit it, could have a material adverse effect on our reputation, business, results of operations and financial condition.
We are subject to product liability and other claims in the ordinary course of business.
Our business involves risk of product liability claims related to providing incorrect product information at the time of purchase, claims for defective containment solutions which may impair drug efficacy and other claims in the ordinary course of business. Furthermore, there may be product liability risks that are unknown or which become known in the future. We may also face claims raised by our present employees for injury deriving from the lifting and handling of loads and the use of heavy machinery, as well as claims raised by our present and past employees for injury and illness from hazardous substances used or present at certain of our facilities. Substantial, complex or extended litigation on any claim could cause us to incur significant costs and distract our management. For example, lawsuits by governmental authorities, employees, shareholders, suppliers, collaborators, distributors, customers,
17
competitors or others could be very costly and substantially disrupt our business. Our exposure to such claims may increase as we seek to increase the geographic scope of our sourcing and sales activities and to the extent that we expand our manufacturing operations. We maintain insurance policies but we cannot assure you that our insurance coverage will be available in all pending or any future cases brought against us. Furthermore, our ability to recover under any insurance is subject to the terms and conditions of such insurance, as well as the financial viability of our and such third parties’ insurers, as well as legal enforcement under the local laws governing these arrangements. Insurance coverage in general or coverage for certain types of liabilities, such as product liability in developing markets, may not be readily available for purchase or cost-effective for us to purchase. Furthermore, many of our insurance policies are subject to deductibles and retentions. Accordingly, we could be subject to uninsured and unindemnified future liabilities requiring us to provide additional reserves to address such liabilities. An unfavorable result in a case for which adequate insurance or indemnification is not available could adversely affect our business, financial condition and results of operations.
Occasionally, we are also involved in disputes, litigation and regulatory matters incidental to and in the ordinary course of our business, including employment matters, commercial disputes, government compliance matters, environmental matters, and other matters arising out of the normal conduct of our business. Where merited, we will vigorously defend ourselves in such matters. There can be no assurance that the impact of any pending or future claims will not be material to our business, financial condition or results of operations.
We are exposed to credit risk on accounts receivable and certain prepayments made in the normal course of business.
Our average day sales outstanding for 2023 has been approximately 67 days, but a number of customers are pushing for longer payment terms (also offering no recourse discounting solutions). A substantial majority of our outstanding trade receivables are not covered by collateral or credit insurance. In addition, we may make advances to suppliers in the normal course of business. While we have procedures to monitor exposure to credit risk on trade receivables and other current assets, there can be no assurance such procedures will effectively limit our credit risk and avoid losses, which could have a material adverse effect on our financial condition and operating results.
We may be required to record a charge to earnings if our goodwill and other amortizable intangible assets, property, plant and equipment or other investments become impaired.
We are required under IFRS to test goodwill for impairment at least annually and to review our goodwill, amortizable intangible assets, property, plant and equipment and other investments, including those acquired through acquisition activity, for impairment when events or changes in circumstance indicate the carrying value may not be recoverable.
Factors that could lead to impairment of goodwill, amortizable intangible assets, property, plant and equipment and other investments, including those acquired through acquisitions, include significant adverse changes in the business climate and actual or projected operating results and declines in the financial condition of our business. We may be required in the future to record additional charges to earnings if our goodwill, amortizable intangible assets, property, plant and equipment or other investments become impaired. Any such charge would adversely impact our financial results.
If relations between China and the United States deteriorate, our business in the United States and China could be materially and adversely affected.
The relationship between China and the United States is subject to periodic tension. Changes in political conditions in the United States and China and changes in the state of China-U.S. relations are difficult to predict and could adversely affect our business.
For instance, the U.S. administration has called for substantial changes to trade agreements and imposed significant increases on tariffs on goods imported into the United States, particularly from China. Other countries have responded similarly, with tariffs on goods entering their countries. We currently have facilities and sell products in China and have invested, and expect to continue investing, in the country, and if the Chinese government makes any changes to its laws or policy concerning foreign ownership of companies or assets located within China, or imposes
18
any significant increases on tariffs on goods imported into or out of China, it could have a significant impact on our business and financial results.
Uncertainties with respect to the People’s Republic of China legal system, including uncertainties regarding the enforcement of laws and sudden and unexpected changes in laws and regulations, could adversely affect us.
Our operations in China are governed by Chinese laws and regulations. The Chinese legal system is based in part on government policies and internal rules, some of which are not published in a timely manner or at all and may have retroactive effect. As a result, we may be in violation of some of these policies and rules, without being aware of such violation. Such unpredictability towards our contractual, property and procedural rights could adversely affect our business and impede our ability to continue our operations in China.
Furthermore, if China adopts more stringent standards with respect to environmental protection or corporate social responsibilities, we may incur increased compliance costs or become subject to additional restrictions in our operations. Intellectual property rights and confidentiality protections in China may also not be as effective as in the United States or other countries.
The Chinese government continues to exercise significant oversight and control over virtually every sector of the Chinese economy through regulation and state ownership. The ability of our Chinese subsidiaries to operate may be impaired by changes in its laws and regulations, including those relating to taxation, land use rights, foreign investment limitations, and other matters.
If the military conflict in Israel and Gaza continues, our business could be materially and adversely affected.
On October 7, 2023, Hamas militants infiltrated Israel’s southern border from the Gaza Strip and conducted a series of attacks. As a result of such attacks, Israel’s security cabinet declared war against Hamas, and Israel launched an aerial bombardment to the Gaza Strip and then also began ground operations in the Gaza Strip, which remain ongoing. It is possible that other regional organizations will join the hostilities as well, resulting in a widening of the conflict, which could negatively impact the global economy.
Furthermore, following Hamas’ attack on Israel and Israel’s security cabinet declaration of war against Hamas, the Houthi movement, which controls parts of Yemen, launched a number of attacks on marine vessels traversing the Red Sea. The Red Sea is a vital maritime route for international trade. These disruptions may impact our ability to receive materials and products from suppliers, to distribute our products in a cost-effective and timely manner and to meet customer demand, all of which could have an adverse effect on our financial condition and results of operations.
There can be no assurance that further unforeseen events impacting the supply chain will not have a material adverse effect on us in the future. Additionally, the impacts that supply chain disruptions have on our third-party manufacturers and suppliers are not within our control. It is not currently possible to predict how long it will take for these supply chain disruptions to cease or ease. Prolonged supply chain disruption could increase raw material and product costs, impact our ability to meet customer demand and result in lost sales, all of which could have a material adverse effect on our business, financial condition and results of operations.
We are monitoring the developing military conflict in Israel and Gaza, but cannot predict whether this situation, which is unfolding in real-time, may escalate and result in material implications for our business. None of our operations are located in Israel or Gaza and currently we do not have any significant customers or suppliers in the region.
If our employees were to engage in a strike or other work stoppage, our business, operating results and financial position could be materially adversely affected.
We employ approximately 5,635 employees, as at December 31, 2023, in multiple jurisdictions (approximately 56% based in Italy, 9% based in Germany, 8% based in Mexico, 6% in each of Slovakia, the U.S. and Denmark, 5% based in Brazil and 4% based in China). A significant portion of our employees in Italy, Germany, Slovakia, Mexico, Denmark and China are covered by collective bargaining arrangements made either at the local or national level in their respective countries. Although we believe that our relations with our employees are satisfactory, no assurance can be given that this will continue. If disputes with our unions arise, or if our workers engage in a strike or other work
19
stoppage, we could incur higher labor costs or experience a significant disruption of operations, which could have a material adverse effect on our business, operating results and financial position.
We are subject to tax laws, tariffs and potential tax audits in multiple jurisdictions that could affect our financial results.
We are subject to tax laws, tariffs and potential tax audits in multiple jurisdictions. The application and interpretation of these laws in different jurisdictions affect our international operations in complex ways and are subject to change, and some changes may be retroactively applied. Our tax liabilities in the different countries where we operate depend, in part, on transfer pricing and administrative charges among us and our subsidiaries. These arrangements require us to make judgments with which tax authorities may disagree, potentially resulting in the assessment of material additional taxes, penalties, interest or other charges to resolve these issues.
Transactions that we have structured in light of current tax rules could have material and adverse consequences for us if tax rules change. Tax audits, changes in tax laws, their application and interpretation or imposition of any new or increased tariffs, duties and taxes could increase our tax burden and materially and adversely affect our sales, profits and financial condition and could have an adverse effect on our business, net assets, or results of operations. Such factors could also cause us to expend significant time and resources and/or cause investors to lose confidence in our reported financial information.
We are exposed to Italian tax risks related to our multinational operations.
We operate in many different jurisdictions throughout the world, through our group companies. Over recent years, tax laws and practice applicable in various countries have become increasingly complex and sophisticated, particularly with respect to cross-border transactions. Italy has historically implemented a number of domestic provisions - including those implementing EU anti-abuse Directives and OECD principles - aimed at facing tax basis erosion schemes and allocation of income between associated enterprises adopted by multinational groups.
Italian Tax authorities are increasingly scrutinizing multinational groups based on these provisions by also enforcing exchange of information instruments in force with foreign tax authorities.
The combination of the above factors may lead to an increased likelihood of tax audits with respect, among other things, to: (i) tax residence, (ii) permanent establishment, (iii) transfer pricing, (iv) Controlled Foreign Company legislation, (v) taxation of dividends and capital gains derived upon interests held in companies located in low-tax Jurisdictions, (vi) withholding tax application on cross-border payments, and (vii) anti-hybrid mismatches. In any such case, depending on the specific circumstances, tax audits and/or tax litigations with the Italian tax authorities could result in tax liabilities and fines and penalties of significant amounts, which could be in excess of the amounts. We provide for in our financial statements for tax liabilities.
The application of indirect taxes could adversely affect our business and results of operations.
The application of indirect taxes, such as sales and use tax, value-added tax, provincial taxes, goods and services tax, business tax and gross receipt tax, to our business is a complex and evolving issue. Significant judgment is required to evaluate applicable tax obligations. As a result, amounts recorded may be subject to adjustments by the relevant tax authorities. In many cases, the ultimate tax determination is uncertain because it is not clear how new and existing statutes might apply to our business. A number of jurisdictions globally have introduced (or are looking to introduce) additional value added tax (or similar tax) calculation requirements as well as additional reporting, record-keeping, collection and remittance obligations on businesses like ours.
There can be no assurance that we will not be a passive foreign investment company (“PFIC”) for U.S. federal income tax purposes. If we are a PFIC for U.S. federal income tax purposes, U.S. Holders holding our shares may be subject to adverse U.S. federal income tax consequences.
A non-U.S. corporation such as the Company will be classified as a PFIC for U.S. federal income tax purposes for any taxable year if either: (i) 75% or more of its gross income for such year consists of certain types of “passive income” or (ii) 50% or more of the value of its assets (determined on the basis of a quarterly average) during such year is attributable to assets that produce or are held for the production of passive income. Whether we are treated as
20
a PFIC is a factual determination that is made on an annual basis after the close of each taxable year. This determination will depend on, among other things, the composition of our income and assets, as well as the value of our assets (which generally will be determined by reference to the public price of our shares, which may fluctuate significantly), from time to time.
Based on the current and anticipated composition of our income, assets and operations and the price of our shares, we do not believe we were a PFIC for U.S. federal income tax purposes for our most recent taxable year and do not expect to be a PFIC for the current taxable year or in foreseeable future years. Nevertheless, there can be no assurance that we will not be a PFIC for any taxable year. If we are treated as a PFIC for any taxable year during which a U.S. Holder (as defined below) holds our shares, such U.S. Holder could be subject to adverse U.S. federal income tax consequences. See “Income Tax Considerations—U.S. Federal Income Tax Considerations – Passive Foreign Investment Company.”
We are subject to restrictive covenants under certain financing agreements, which could impair our ability to run our business.
We have in place a number of financing agreements which include covenants (such as negative covenants that would restrict our ability to distribute dividends and exceed certain indebtedness ratios) which may restrict our ability to operate our business. Our failure to comply with these covenants, including as a result of events beyond our control, could result in a default or event of default that could materially and adversely affect our financial condition and results of operations. For additional information on applicable regulations see “Operating and Financial Review and Prospects—Liquidity and Capital Resources.”
The indemnification provisions of acquisition agreements by which we have acquired companies or businesses may not fully protect us, and we may face unexpected liabilities.
Certain of the acquisition agreements by which we have acquired companies or businesses require the former owners to indemnify us against certain liabilities related to the operation of the company or business before we acquired it. In most of these agreements, however, the liability of the former owners is limited, and certain former owners may be unable to meet their indemnification responsibilities.
While we are protected by representation and warranties and related indemnification in relation to such acquisitions, these may not be enough to cover our exposure if a significant liability arose in connection with any acquisition agreement. We cannot assure you that these indemnification provisions will protect us fully or at all, and as a result we may face unexpected liabilities that adversely affect our business, financial condition and results of operations.
Our business depends on our ability to use and access information systems, and any failure to successfully maintain these systems or implement new systems to handle our changing needs could materially harm our operations.
We depend on standardized procedures and multiple information systems for our operations, customer service and quality and safety procedures.
Furthermore, we rely on information technology systems to process, transmit, store and protect electronic information, including confidential customer, supplier, employee or other business information. Through our online platform, we collect and store confidential information that website users provide to us when submitting queries or job applications.
We use commercially available third-party technology solutions, software and software systems with some proprietary configurations. We also store data using third-party cloud services. Our information systems may be subject to damage or interruption from power outages, computer and telecommunications failures, computer viruses, security breaches, vandalism, catastrophic events, natural disasters, terrorist attacks, hackers and other security issues as well as human error. If our information systems are damaged, fail to work properly or otherwise become unavailable, we may incur substantial costs to repair or replace them, and we may experience a loss of critical information, customer disruption and interruptions or delays in our ability to perform essential functions and implement new and innovative services. If the cloud service providers we use were to experience unplanned downtime,
21
delays or other issues delivering data to our information technology systems, it could adversely impact business operations. The compromising of our information systems or those with which we interact could harm our reputation and expose us to regulatory actions and claims from customers and other persons, any of which could adversely affect our business, financial condition and results of operations.
In addition, we may not have the necessary resources to enhance existing information systems or implement new systems where necessary to handle our increasing volume and/or our changing needs, and we may experience unanticipated delays, complications and expenses in implementing and integrating our systems. Any interruptions in operations would adversely affect our ability to properly allocate resources and timely deliver our products, which could result in customer dissatisfaction. We currently rely on certain legacy systems that are no longer supported by their respective manufacturers, with only a small number of current employees able to maintain these systems. Any failure of these systems could have a business impact. The failure to successfully implement and maintain information systems could have an adverse effect on our ability to obtain new business, retain existing business and maintain or increase our sales and profit margins, any of which could adversely affect our business, financial condition and results of operations.
Cyber security risks and the failure to maintain the confidentiality, integrity and availability of our computer hardware, software and internet applications and related tools and functions, could result in damage to our reputation, data integrity and/or subject us to costs, fines or lawsuits under data privacy or other laws or contractual requirements.
The integrity and protection of the data we hold is critical to our business. The regulatory environment governing information, security and privacy laws is increasingly demanding and continues to evolve. Implementing and maintaining compliance with applicable security and privacy regulations may increase our operating costs and/or adversely impact our ability to market our products and services to customers. Although our computer and communications hardware are protected through physical and software safeguards, they are still vulnerable to fire, storm, flood, power loss, earthquakes, telecommunications failures, physical or software break-ins, software viruses and similar events. These events could lead to the unauthorized access, disclosure and use of non-public information. We could be subject to risks caused by misappropriation, misuse, leakage, falsification, system malfunction or intentional or accidental release or loss of information maintained in our information systems and networks and those of our OEM suppliers, including our cloud service providers.
The techniques used by criminal elements to attack computer systems are sophisticated, change frequently and may originate from less regulated and remote areas of the world. Due to the evolving nature of cyber threat actors and the frequency and sophistication of the cyber activities they carry out, the determination of the severity and potential impact of a cyber incident may not become apparent for a substantial period of time following discovery of the incident and we may not be able to address these threats proactively or implement adequate preventative measures. For example, our business could be harmed if we are faced with or exposed to unauthorized use of generative artificial intelligence in open structured systems, which can result in inadvertent disclosure of protected information.
Although we take measures to protect data from unauthorized access, use or disclosure, our information technology and infrastructure may be vulnerable to attacks by hackers or viruses or breached due to employee error, malfeasance or other malicious or inadvertent disruptions.
If our computer systems are compromised, or if we are unable to maintain reliable information technology systems and appropriate controls with respect to global data privacy and security requirements and prevent data breaches, we could be subject to fines, damages, litigation and enforcement actions. We may also suffer business consequences such as reputational damage, or lose trade secrets, the occurrence of which could harm our business.
In addition, we may also be adversely affected if our third-party vendors, customers advisors or agents are subject to a successful cyber-attack or other information security event. Any such breach. or interruption or other loss of information could compromise our networks, and the information stored there could be accessed by unauthorized parties, manipulated, publicly disclosed, lost or stolen.
Cybersecurity insurance may not cover all losses and damages from such events and may limit the ability to maintain or obtain sufficient insurance coverage in the future.
22
We collect, process, store, use and share data, some of which contains personal data, which subjects us to complex and evolving governmental regulation and other legal obligations related to data privacy, data protection, information security and other matters, which are subject to change and uncertain interpretation.
EU Member States, the United Kingdom and many other non-US jurisdictions have adopted statutes and/or regulations concerning privacy and data protection and requiring notification of personal data security breaches if certain thresholds are met. For example, the EU adopted the General Data Protection Regulation (“GDPR”), which became effective in 2018, and the UK transposed the GDPR into national law (“UK GDPR”) following the exit of the United Kingdom from the European Union, which became effective in 2021 (collectively, Applicable Data Protection Laws). The Applicable Data Protection Laws impose strict requirements on controllers and processors of personal data in the European Economic Area, or EEA and the United Kingdom, including, for example, higher standards for obtaining consent from individuals to process their personal data, more robust disclosures to individuals and a strengthened individual data rights regime and shortened timelines for data breach notifications.
Failure to comply with the GDPR or UK GDPR may result in monetary penalties of up to €20.0 million or 4% of an undertaking’s total worldwide annual turnover of the previous financial year, whichever is higher. Given the EU GDPR and UK GDPR are separate regimes, fines could arise under each in respect of a single incident, to the extent it affects EEA and UK personal data. In addition to fines, a breach of the GDPR or UK GDPR may result in regulatory investigations, reputational damage, orders to cease/change our data processing activities, enforcement notices, assessment notices (for a compulsory audit) or civil claims (including class actions).
The UK GDPR is in a currently substantially unvaried form from the GDPR, however, it is likely to be subject to divergence from the GDPR over time as demonstrated by the Data Protection and Digital Information (No. 2) Bill which is under the United Kingdom Parliament’s consideration and expected to come into force in 2024. We may therefore be subject in the future to separate and additional data protection obligations to those that we are already subject to. This may result in additional costs and may necessitate changes to our business practices, which in turn may compromise our growth strategy and otherwise adversely affect our business, reputation, legal exposures, financial condition and results of operations. In recent years, the United States and European lawmakers and regulators have expressed concern over electronic marketing and the use of third-party cookies, web beacons and similar technology for online behavioral advertising. In the European Union, marketing is defined broadly to include any promotional material and the rules specifically on e-marketing are currently set out in the ePrivacy Directive which will be replaced by a new ePrivacy Regulation. In the EEA and in the UK under national laws derived from the e-Privacy Directive, informed consent is required for the placement of a cookie or similar technologies on a user’s device and for e-marketing. The GDPR and UK GDPR also impose conditions on obtaining valid consent for cookies, such as a prohibition on pre-checked consents and a requirement to ensure separate consents are sought for each type of cookie or similar technology. Recent European court decisions and regulators’ recent guidance are driving increased attention to cookies and tracking technologies and the online behavioral advertising ecosystem. This could lead to substantial costs, require significant systems changes, limit the effectiveness of our marketing activities, divert the attention of our technology personnel, adversely affect our margins, increase costs and subject us to additional liabilities. In addition, regulation of cookies and similar technologies, and any decline of cookies or similar online tracking technologies as a means to identify and potentially target users, may lead to broader restrictions and impairments on our marketing and personalization activities and may negatively impact our efforts to understand users. Finally, the current national laws that implement the e-Privacy Directive are highly likely to be replaced across the EU (but not the UK) with a EU regulation known as the e-Privacy Regulation which, though still in development, will if adopted, impose new obligations on the use of personal data in the context of electronic communications, particularly in relation to online tracking technologies, and significantly increase regulators’ ability to impose fines for non-compliance. This again introduces the possibility we will be subject to, and required to comply with, a separate and additional legal regime with respect to data privacy, which may result in additional costs and may necessitate changes to our business practices, which in turn may compromise our growth strategy and otherwise adversely affect our business, reputation, legal exposures, financial condition and results of operations.
On August 20, 2021, the Standing Committee of the National People's Congress of the People's Republic of China promulgated the so-called Personal Information Protection Law (the “PIPL”), which entered into force on November 1, 2021. The PIPL, regarded as China’s version of the GDPR, aims at protecting the personal information rights and interests ensuring the orderly and free flow of personal information in accordance with the law, and promotes the reasonable use of personal information. The PIPL regulates how business operators may collect, use, process, share,
23
and transfer personal information in China and supplements the existing data protection regime previously established by the Cybersecurity Law (“CSL”) and other fragmented national guidelines. Under the PIPL, personal information handlers must adopt necessary measures to safeguard the security of personal information. The PIPL further mandates that, in case of violations, the business operators can receive orders of rectification, suspension, termination of provision of services, or confiscation of illegal income.
There are also numerous U.S. federal and state laws and regulations related to the privacy and security of personal information.
For example, the California Consumer Privacy Act of 2018, which came into effect in 2020, requires disclosures of our privacy practices to California consumers and affords such consumers certain rights, such as the right to opt out of the sale of their personal data. The California Privacy Rights Act of 2020 amended the California Consumer Privacy Act of 2018 which came into effect on January 1, 2023, imposes additional data protection obligations on companies doing business in California and grants California consumers additional rights, potentially resulting in further complexity for our compliance efforts.
Additionally, the Gramm-Leach-Bliley Act of 1999 (along with its implementing regulations) (the “GLBA”) restricts certain collection, processing, storage, use and disclosure by covered companies of certain personal information, requires notice to individuals of privacy practices and provides individuals with certain rights to prevent the use and disclosure of certain non-public or otherwise legally protected information. The GLBA also imposes requirements regarding the safeguarding and proper destruction of personal information through the issuance of data security standards or guidelines.
State laws are changing rapidly (with at least ten such states (in addition to California) enacting comprehensive privacy laws scheduled to take effect starting in 2023, and privacy bills proposed in a number of other states in varying stages of the legislative process), and there is discussion in Congress of a new comprehensive federal data protection law to which we would become subject if it were enacted, which may lead to additional complexity for our compliance efforts and new restrictions regarding how we use data, and which may expose us to potential legal risks. The evolving regulatory landscape may require additional investment of resources in our compliance programs, impact our strategies and the availability of information useful for our business, and could otherwise result in increased compliance costs or changes in our business practices and policies.
The cross-border data transfer landscape globally (including in the EEA, United Kingdom and United States) is continually evolving, and other countries outside of Europe have enacted or are considering enacting cross-border data transfer restrictions and laws requiring data localization, which may affect our ability to process or transfer personal data from Europe or elsewhere. The EU's adequacy decision with respect to the UK, which allows the continued flow of personal data from the EU to the UK following Brexit, will be regularly reviewed and may be revoked if the UK diverges from its current adequate data protection laws. The UK has developed its own international data transfer agreement, which was implemented in March 2022. GDPR and UK GDPR, as well as other statutes and/or regulations concerning privacy and data protection, increase compliance obligations, affect collection, processing, retention and transfer of personal data and the reporting of personal data security breaches, and provide for increased penalties for non-compliance. On July 11, 2023, the European Commission entered into force its adequacy decision for the EU-US Data Privacy Framework (a new framework for transferring personal information from the EEA to the United States), having determined that such framework ensures that the protection of personal information transferred from the EEA to the US will be comparable to the protection offered in the EU. The UK has also approved a UK extension to the EU-US Data Privacy Framework, which were laid before Parliament in September 2023 and come into force on October 12, 2023. However, this decision will likely face legal challenges and ultimately may be invalidated by the Court of Justice of the European Union (“CJEU”).
We are also reliant on certain manual processes for collecting and processing data, and any failures in these processes or failure to handle the data collected in accordance with relevant regulations could lead to enforcement actions. Complying with all applicable laws, regulations, standards and obligations relating to data privacy, security and transfers may cause us to incur substantial operational costs or require us to modify our data processing practices and processes. Government enforcement actions can be costly and interrupt the regular operation of our business, and data breaches or violations of data privacy laws can result in significant fines, reputational damage and civil lawsuits, any of which may adversely affect our business, financial condition and results of operations. We may not be able to respond quickly or effectively to regulatory, legislative and other developments, and these changes may in turn impair
24
our ability to commercialize our products or increase our cost of doing business. In addition, if our practices are not consistent or viewed as not consistent with legal and regulatory requirements, including changes in laws, regulations and standards or new interpretations or applications of existing laws, regulations and standards, we may become subject to audits, inquiries, whistleblower complaints, adverse media coverage, investigations, loss of export privileges, severe criminal or civil sanctions or reputational damage. Any of the foregoing could have an adverse effect on our competitive position, business, financial condition, results of operations and prospects.
The uncertain effects of climate change and potential climate change legislation could lead to business interruption, significantly increased costs and/or other adverse consequences to our business.
Climate change and potential climate change legislation may present risks to our operations, including business interruption, significantly increased costs and/or other adverse consequences to our business. Some of the potential impacts of climate change to our business include physical risks to our facilities, water and energy supply limitations or interruptions, disruptions to our supply chain and impairment of other resources. In addition, if legislation or regulations are enacted or promulgated in the U.S., Europe or Asia or any other jurisdictions in which we do business that limit or reduce allowable greenhouse gas emissions and other emissions, such restrictions could have a significant effect on our operating and financial decisions, including those involving capital expenditures to reduce emissions, and our results of operations. Our manufacturing operations may not be able to operate as planned if we are not able to comply with new legal and regulatory legislation around climate change, or it may become too costly to operate in a profitable manner. Additionally, suppliers’ added expenses could be passed on to us in the form of higher prices and we may not be able to pass on such expenses to our customers through price increases.
Risks Relating to our Intellectual Property
Our trade secrets may be misappropriated or disclosed, and confidentiality agreements with directors, employees and third parties may not adequately prevent disclosure of trade secrets and protect other proprietary information.
In addition to registered intellectual property rights, we rely on trade secrets and confidential know-how to protect our technology, especially because we believe that patent protection alone would not be sufficient to protect our business. However, trade secrets and confidential know-how are difficult to protect, and we have limited control over the protection of trade secrets and confidential know-how used by our licensors, collaborators and suppliers.
To protect this type of information against disclosure or appropriation by competitors, our usual practice is to require our directors, employees, consultants, contractors and advisors to enter into confidentiality agreements and, if applicable, material transfer agreements, consulting agreements or other similar agreements with us prior to beginning research or disclosing proprietary information. Moreover, we put in place appropriate procedures to identify confidential material and restrict access to documentation. However, current or former employees, consultants, contractors and advisers may unintentionally or willfully disclose our confidential information to competitors, we have entered into, and may in the future enter into additional, collaborations with our competitors, and confidentiality agreements may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known to our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Enforcing a claim that a third party obtained illegally and is using trade secrets and/or confidential know-how is expensive, time consuming and the outcome is unpredictable, and the enforceability of confidentiality agreements may vary from jurisdiction to jurisdiction.
Moreover, if any of our trade secrets and confidential know-how were to be lawfully obtained or independently developed by a competitor or other third party, we would have no right to prevent them from using that technology or information to compete with us. In some cases, we have entered into joint development agreements with our competitors that necessitate the sharing of certain trade secrets with these competitors. Given that our competitive position is based, in part, on our know-how and trade secrets, a competitor’s knowledge of our trade secrets or other unauthorized use or disclosure could impair our competitive position and may have an adverse effect on our business and results of operations.
25
If we are unable to obtain and maintain patent protection for our technology, products and potential products, or if the scope of the patent protection obtained is not sufficiently broad, we may not be able to compete effectively in our markets.
Our success depends in part on our ability to secure and maintain patent protection with respect to our technology, current products and potential products, and any future potential products and technology we may develop. We seek to protect our proprietary position by filing or collaborating with our licensors to file patent applications related to our proprietary technologies, products and potential products.
The patent prosecution process is expensive, time consuming and complex, and we may not be able to file, prosecute, maintain, defend, enforce or license all necessary or desirable patents at a reasonable cost or in a timely manner in all desirable jurisdictions. As a result, we may not be able to prevent competitors or other third parties from developing and commercializing competitive products in all such fields and jurisdictions.
It is possible that we will fail to identify patentable aspects of our research and development output or fail to take the necessary steps to seek patent protection before it is too late to obtain patent protection. We may not have the right to control the preparation, filing, and prosecution of patent applications, or to maintain the rights to patents licensed from third parties. Therefore, these patents and patent applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our relevant proprietary products and technology, including current products, potential products, and any future potential products we may develop, in whole or in part. Our existing patents may have been issued with claims that fail to cover our relevant proprietary products and technology, including current products, potential products and any future potential products we may develop, in whole or in part. In addition, our existing patents and any future patents we obtain may not be sufficiently broad to prevent others from using our technology or from developing competing products and technologies. Patents may not be granted for a number of reasons, including known or unknown prior art, deficiencies in the patent application or the lack of novelty or the underlying invention or technology. In addition, publications of discoveries in scientific literature often lag behind the actual discoveries, and patent applications in certain jurisdictions are not published until 18 months after filing or in some cases, at all. Therefore, we cannot be certain that we or our licensors were the first to make or file the inventions claimed in our owned or licensed patents or pending patent applications.
Even if patents do successfully issue and even if such patents cover our current products, current potential products and any future potential products we may develop, third parties may challenge their validity, ownership, enforceability or scope, which may result in such patents being narrowed, invalidated, or held unenforceable or circumvented.
We may become involved in proceedings challenging our owned or licensed patent rights, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or could limit the duration of the patent protection of our technology, products and potential products. Such proceedings also may result in substantial costs and require significant time from our management and employees, even if the eventual outcome is favorable to us. In addition, the issuance of a patent does not give us the right to practice the patented invention. Third parties may have blocking patents that could prevent us from marketing our products, if approved, or practicing our own patented technology. Our competitors may also be able to circumvent our patents by developing similar or alternative potential products in a non-infringing manner.
Any of the foregoing could have an adverse effect on our competitive position, business, financial condition, results of operations and prospects.
We depend in part on proprietary technology licensed from others. If we lose our existing licenses or are unable to acquire or license additional proprietary rights from third parties, we may not be able to continue developing our potential products.
We are a party to certain license agreements for certain intellectual property and proprietary technology, and we may enter into additional agreements, including license agreements, with other parties in the future that impose certain obligations on us. If we fail to comply with our obligations to our licensors or any of our other current or future collaborators, our counterparties may have the right to terminate these agreements, in which event we might not be able to develop, manufacture or market any product, potential product or other technology that is covered by these
26
agreements, which could adversely affect the value of the potential product being developed under any such agreement, or we may face claims for monetary damages or other penalties under these agreements. Termination of these agreements or reduction or elimination of our rights under these agreements may result in us having to negotiate new or reinstated agreements with less favorable terms, or cause us to cease or experience significant delays in the development and commercialization of our products, potential products or technologies and, our competitors or other third parties could have the freedom to market products and technologies identical or competitive to ours.
We may rely on third parties from whom we license proprietary technology to file and prosecute patent applications and maintain patents and otherwise protect the intellectual property we license from them. We may have limited control over these activities or any other intellectual property that may be related to our in-licensed intellectual property. We may have limited control over the manner in which our licensors initiate an infringement proceeding against a third-party infringer of the intellectual property rights, or defend certain of the intellectual property that may be licensed to us.
The growth of our business may depend in part on our ability to acquire or in-license additional proprietary rights. We may be unable to acquire or in-license any relevant third-party intellectual property rights that we identify as necessary or important to our business operations. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all, which would harm our business. In that event, we may be required to expend significant time and resources to redesign our products, potential products or technologies or the methods for manufacturing them or to develop or license replacement technology, all of which may not be feasible on a technical or commercial basis, which could adversely impact our business, financial condition, results of operations and prospects.
Disputes may arise regarding intellectual property subject to a license agreement and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could affect what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our diligence, financial or other obligations under the relevant agreement, or we may face claims for monetary damages or other penalties under these agreements. Moreover, disputes may also arise over the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors or licensees and us and our partners. If disputes over intellectual property that we have licensed or any other dispute described above related to our license agreements prevent or impair our ability to use and enforce such intellectual property or maintain our licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected products, potential products or technologies. Any of the foregoing could have an adverse effect on our competitive position, business, financial condition, results of operations and prospects.
Third parties may assert claims against us alleging infringement, misappropriations or other violations of their patents and proprietary rights, or we may need to become involved in lawsuits to defend or enforce our patents, either of which could result in substantial costs or loss of productivity, delay or prevent the development and commercialization of our products and potential products, or prohibit our use of proprietary technology or sale of products.
Our commercial success depends, in part, upon our ability to develop, manufacture, market and sell our products and other technologies without alleged or actual infringement, misappropriation or other violation of the patents and proprietary rights of third parties. However, our research, development and commercialization activities may be subject to claims that we infringe, misappropriate or otherwise violate patents or other intellectual property rights owned or controlled by third parties. The various markets in which we operate can be subject to litigation regarding patents and other intellectual property rights.
For example, our third-party collaborators may not properly obtain, maintain, enforce or defend our intellectual property or proprietary rights or may use or misappropriate our proprietary information in such a way that could jeopardize or invalidate our intellectual property rights or expose us to potential litigation. Our competitors have made substantial investments in patent portfolios and competing technologies, and may have applied for or obtained or may in the future apply for or obtain, patents that will prevent, limit or otherwise interfere with our ability to make, use and sell our products. In addition, patent holding companies that focus solely on extracting royalties and settlements by enforcing patent rights may target us.
27
We may be subject to third-party claims including patent infringement or similar adversarial proceedings or litigation in various jurisdictions. Even if we believe such claims are without merit, a court of competent jurisdiction could hold that these third-party patents are valid, enforceable and infringed, and the holders of any such patents may be able to block our ability to commercialize the applicable product or potential product unless we obtained a license under the applicable patents, or until such patents expire or are finally determined to be invalid or unenforceable. Third parties may obtain patents in the future and claim that use of our technologies, products and potential products infringes upon these patents. Additionally, because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that our products, potential products or technologies may infringe. If any third-party patents issued from such applications were held by a court of competent jurisdiction to cover aspects of our products, potential products or technologies, the holders of any such patents may be able to prohibit our commercialization of the applicable product, potential product or technology until such patent expires or is finally determined to be invalid or unenforceable or unless we obtained a license.
In addition, defending such claims could cause us to incur substantial expenses and, if we fail, could cause us to pay substantial damages if we are found to be infringing a third party’s patent rights. Further, if a patent infringement suit is brought against us, our development, manufacturing or sales activities relating to the product, potential product or technology that is the subject of the suit may be delayed or terminated, as parties making claims against us may obtain injunctive or other equitable relief. As a result of patent infringement claims, or in order to avoid potential infringement claims, we may choose to seek, or be required to seek, a license from the third party, which may require payment of substantial royalties or fees, or require us to grant a cross-license under our intellectual property rights. These licenses may not be available on reasonable terms or at all. Even if a license can be obtained on reasonable terms, the rights may be nonexclusive, which would give our competitors access to the same intellectual property rights. If we are unable to enter into a license on acceptable terms, we could be prevented from commercializing one or more of our products, potential products or technologies, or forced to modify such products or potential products, or to cease some aspect of our business operations, which could harm our business significantly. We might also be forced to redesign or modify our products, potential products or technologies so that we no longer infringe the third-party intellectual property rights, which may result in significant cost or delay to us, or such redesign or modification could be impossible or technically not viable.
Even if we were ultimately to prevail, any of these events could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business, force us to face negative publicity, adversely impact prospective customers or prohibit us from manufacturing, importing, marketing or otherwise commercializing our products, potential products, services and technology. In addition, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
There could also be public announcements of the results of hearings, motions or other interim proceedings or developments, and if securities analysts or investors view these announcements in a negative light, the price of our ordinary shares could be adversely affected. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources, adversely affecting our ability to compete in the marketplace.
We may become involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time consuming and unsuccessful and have an adverse effect on the success of our business.
Competitors or other third parties may infringe, misappropriate or otherwise violate our patents or other intellectual property. In addition, our third-party collaborators may use or misappropriate our intellectual property and proprietary information in such a way that could jeopardize our ownership and intellectual property rights.
If we or one of our licensors were to initiate legal proceedings against a third party to enforce a patent covering one of our products or potential products, the defendant could counterclaim that our patent is invalid or unenforceable. In patent litigation in certain countries, defendant counterclaims alleging invalidity or unenforceability are commonplace. Third parties may initiate invalidity proceedings even in the absence of infringement proceedings. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements. Interference or derivation proceedings provoked by third parties or brought by us or declared by the relevant patent authority may
28
be necessary to determine the priority of inventions with respect to our patents or patent applications. The outcome of proceedings involving assertions of invalidity and unenforceability during patent litigation is unpredictable.
If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our products, potential products and other technology, which may allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or could require us to obtain license rights from the prevailing party in order to be able to manufacture or commercialize our products, potential products or technologies without infringing third-party patent rights. Even if a defendant does not prevail on a legal assertion of invalidity or unenforceability, our patent claims may be construed in a manner that would limit our ability to enforce such claims against the defendant and others. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon, misappropriating or otherwise violating our intellectual property rights. Even if we were to successfully assert our patents or other intellectual property rights, or to settle at an early stage, such litigation could burden us with substantial unanticipated costs or a court may not award remedies that sufficiently compensate us for our losses. The impact of public announcements of the results of hearings related to such awards on our business may be uncertain. Our patents and other intellectual property rights also will not protect our technology, products and potential products if competitors design around our protected technology, products and potential products without infringing our patents or other intellectual property rights.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
We rely on external law firms, their extended network of partners worldwide and their internal check procedures for patent maintenance and prosecution. In the event that we or our licensors fail to maintain the patents and patent applications covering our products and potential products or if we or our licensors otherwise allow our patents or patent applications to be abandoned or lapse, it could create opportunities for competitors to enter the market, which would hurt our competitive position and could impair our ability to successfully commercialize our products.
We enjoy only limited geographical protection with respect to certain patents, and we may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents covering our technology, products and potential products in all countries throughout the world would be prohibitively expensive, and even in countries where we have sought protection for our intellectual property, such protection can be less extensive than those in Europe and the United States.
Competitors may use our and our licensors’ technologies in jurisdictions where we have not obtained patent protection or licensed patents to develop their own products and, further, may export otherwise infringing products to territories where we and our licensors have patent protection, but where enforcement is not as strong as that in the European Union or the United States. These products may compete with our products, and our or our licensors’ patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many jurisdictions have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many jurisdictions limit the enforceability of patents against government agencies or government contractors. In these jurisdictions, the patent owner may have limited remedies, which could materially diminish the value of such patents. If we or any of our licensors are forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be adversely impacted, which could have a material adverse effect on our business.
The legal system in certain foreign jurisdictions, particularly those in certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection. Proceedings to enforce our patent and other intellectual property rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents and other intellectual property rights at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a commercial advantage from the intellectual property that we develop or license. Any of the
29
foregoing could have an adverse effect on our competitive position, business, financial condition, results of operations and prospects.
We may be subject to claims that our employees, consultants, independent contractors or collaborators have wrongfully used or disclosed confidential information of their former employers or other third parties, and we may be subject to claims asserting ownership of what we regard as our own intellectual property.
We do and may employ individuals who were previously employed at universities or other life sciences companies, including our licensors, competitors or potential competitors. Although we seek to protect our ownership of intellectual property rights by ensuring that our agreements with our employees, consultants, collaborators, independent contractors and other third parties with whom we do business include provisions requiring such parties to assign rights in inventions to us and to not use the know-how or confidential information of their former employer or other third parties, we may be subject to claims that we or our employees, consultants, collaborators or independent contractors have inadvertently or otherwise used or disclosed know-how or confidential information of their former employers or other third parties, or that former employers or other third parties have an ownership interest in our patents. Litigation may be necessary to defend against these claims, and if we fail, in addition to paying monetary damages, we may lose valuable personnel or intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property, which could result in customers seeking other sources for the technology, or ceasing from doing business with us. Moreover, any such litigation or the threat thereof may adversely affect our reputation, our ability to form strategic alliances, engage with scientific advisors or hire employees or consultants, any of which could adversely affect our business, including in terms of substantial cost, reputational loss and/or a distraction to our management and other employees.
If conflicts arise between us and our collaborators or strategic partners, these parties may act in a manner adverse to us and could limit our ability to implement our strategies and protect our intellectual property rights.
If conflicts arise between our corporate or academic collaborators or strategic partners and us, the other party may act in a manner adverse to us and could limit our ability to implement our strategies and protect our intellectual property rights. Our collaborators or strategic partners may have or may, in the future, develop, either alone or with others, products in related fields that are competitive with the products we have or may develop. In addition, our collaborators or strategic partners may use our intellectual property and proprietary information in such a way that could jeopardize our ownership and intellectual property rights. Competing products, either developed by the collaborators or strategic partners or to which the collaborators or strategic partners have rights, may result in the withdrawal of partner support for our products.
Our collaborators or strategic partners also could preclude us from entering into collaborations with their competitors, fail to obtain timely regulatory approvals, terminate their agreements with us prematurely, fail to devote sufficient resources to the development and commercialization of products, use our intellectual property and proprietary information in such a way that could jeopardize our ownership and intellectual property rights, or merge with or be acquired by a third party who may do any of these things. Any of the foregoing could harm our development and commercialization efforts and materially adversely affect our business.
If our trademarks and trade names are not adequately protected, we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Although we currently own trademark registrations and have trademark applications pending, it may be possible that some trademarks may be the subject of a governmental or third-party objection, which could prevent the registration or maintenance of the same. We cannot assure you that any currently pending trademark applications or any trademark applications we may file in the future will be approved. If we are unsuccessful in obtaining trademark protection for our primary brands, we may be required to change our brand names; additionally, if competitors try to adopt trade names or trademarks similar to ours, this might impede our ability to build brand identity and possibly lead to market confusion, adversely affecting our business in the long-term. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our unregistered trademarks or trade names.
30
Our efforts to enforce or protect our proprietary rights related to trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely impact our financial condition or results of operations.
Risks Relating to Our Shares
The price of our ordinary shares may be volatile and may fluctuate due to factors beyond our control.
The market price of our ordinary shares may fluctuate significantly due to a variety of factors, including:
These and other market and industry factors may cause the market price and demand for our ordinary shares to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from readily selling their ordinary shares and may otherwise negatively affect the liquidity of our ordinary shares. In addition, the stock market in general, and pharmaceutical and biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. In the past, when the market price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation against the issuer. If any of the holders of our ordinary shares were to bring such a lawsuit against us, we could incur substantial costs defending the lawsuit and the attention of our senior management would be diverted from the operation of our business. Any adverse determination in litigation could also subject us to significant liabilities.
The dual class structure of our shares may adversely affect the value and trading market for the ordinary shares.
Our shares carry different voting rights depending on their class. Holders of ordinary shares are entitled to one vote per share, while holders of Class A shares (held solely by Stevanato Holding S.r.l. or held in treasury by the Company) are entitled to three votes per share. Under no circumstances the ordinary shares can be converted into Class A shares. We cannot predict whether our dual class structure will result in a lower or more volatile market price for our ordinary shares or in adverse publicity or other adverse consequences. For example, certain index providers such as S&P Dow Jones and FTSE Russell have announced restrictions on including companies with multiple-class share structures in certain of their indexes.
31
In addition, several stockholder advisory firms have announced their opposition to the use of multiple class structures.
As a result, the dual class structure of our shares may cause stockholder advisory firms to publish negative commentary regarding our corporate governance practices or otherwise seek to cause us to change our capital structure. Any such exclusion from indices or any actions or publications by stockholder advisory firms critical of our corporate governance practices could adversely affect the value and trading market for our ordinary shares.
If securities or industry analysts do not publish research, or publish inaccurate or unfavorable research, about our business, the price of our ordinary shares and our trading volume could decline.
The trading market for our ordinary shares will depend in part on the research and reports that securities or industry analysts publish about us and our business. Securities and industry analysts do not currently, and may never, publish research on us. If no or not enough securities or industry analysts commence coverage on us, the trading price for our ordinary shares would likely be negatively affected. In the event securities or industry analysts initiate coverage, if one or more of the analysts who cover us downgrade our ordinary shares or publish inaccurate or unfavorable research about our business, the price of our ordinary shares would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, demand for our ordinary shares could decrease, which might cause the price of our ordinary shares and trading volume to decline.
Our voting control is concentrated.
Stevanato Holding S.r.l., our controlling shareholder and holding company of the Stevanato family, exercises a significant majority of the voting power with respect to our outstanding shares because of the multiple voting shares that it holds. Class A Shares are entitled to three votes per share, and ordinary shares are entitled to one vote per share. Excluding treasury shares (which voting right is suspended), Stevanato Holding S.r.l. holds 95.20% of the voting rights of the Company.
As a result, the Company qualifies as a “controlled company” pursuant to the NYSE listing rules and, therefore, Stevanato Holding S.r.l. potentially has the ability to control the outcome of matters submitted to our shareholders for approval, including the election and removal of directors and any arrangement or sale of all or substantially all of our assets. This concentrated control could delay, defer or prevent a change of control, arrangement or merger or sale of all or substantially all of our assets that our other shareholders may support. Conversely, this concentrated control could allow the holder of the class A multiple voting shares to consummate a transaction that our other shareholders do not support. In addition, the holder of the class A multiple voting shares may make long-term strategic investment decisions and take risks that may not be successful and/or may seriously harm our business.
Future sales, or the possibility of future sales, of a substantial number of our shares could adversely affect the price of our ordinary shares.
Future sales of a substantial number of our shares, or the perception that such sales will occur, could cause a decline in the market price of our ordinary shares.
We have ordinary shares outstanding. The ordinary shares are freely tradable without restriction under the Securities Act, except for any of our shares that may be held or acquired by our directors, executive officers and other affiliates, as that term is defined in the Securities Act, which are restricted securities under the Securities Act. Restricted securities may not be sold in the public market unless the sale is registered under the Securities Act or an exemption from registration is available.
In the future, we may also issue our securities if we need to raise capital in connection with a capital raise or acquisition. The amount of securities issued in connection with a capital raise or acquisition could constitute a material portion of our then-outstanding shares.
32
The rights of our shareholders may differ from the rights typically offered to shareholders of a U.S. corporation.
We are incorporated as a joint stock company (società per azioni) under Italian law. The rights of holders of our shares and, therefore, certain of the rights of holders of shares, are governed by Italian law, including certain provisions of the Italian Civil Code (the “Italian Civil Code”) and by our articles of association.
These rights differ in certain respects from the rights of shareholders in typical U.S. corporations. See “Description of Share Capital—Differences in Corporate Law” in this annual report for a description of the principal differences between the provisions of the Italian Civil Code applicable to companies that are listed on a regulated market (società che fanno ricorso al mercato del capitale di rischio) and, for example, the Delaware General Corporation Law relating to shareholders’ rights and protections.
Claims of U.S. civil liabilities may not be enforceable against us.
We are incorporated under Italian law. Most of our assets are located outside the United States. The majority of our management and board of directors reside outside the United States. As a result, it may not be possible for investors to effect service of process within the United States upon such persons or to enforce judgments obtained in U.S. courts against them or us, including judgments predicated upon the civil liability provisions of the U.S. federal securities laws.
No assurance can be given that we will continue to pay or declare dividends.
We have historically paid dividends during the last three years. However, there can be no assurance that we will pay or declare dividends in the future. The actual declaration and payment of future dividends, the amount of any such dividends, and the establishment of record and payment dates, if any, are subject to determination by our Board of Directors each quarter after its review of the current strategy, applicable debt covenants and financial performance and position, among other things. Our declaration and payment of future dividends is subject to risks and uncertainties, including: deterioration of our financial performance or position; inability to declare a dividend in compliance with applicable laws or debt covenants; an increase in our cash needs or decrease in available cash; and the business judgment of the board of directors that a declaration of a dividend is not in our best interest.
As a foreign private issuer, we are exempt from a number of rules under the Exchange Act, we are permitted to file less information with the SEC than domestic companies, and we are permitted to follow home country practice in lieu of the listing requirements of NYSE, subject to certain exceptions. Accordingly, there may be less publicly available information concerning us than there is for issuers that are not foreign private issuers.
As a foreign private issuer, we are exempt from certain rules under the Exchange Act, including certain disclosure and procedural requirements applicable to proxy solicitations under Section 14 of the Exchange Act, our board of directors, officers and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act, and we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as companies whose securities are registered under the Exchange Act but are not foreign private issuers. Foreign private issuers are also not required to comply with Regulation FD, which restricts the selective disclosure of material non-public information. Accordingly, there may be less publicly available information concerning us than there is for companies whose securities are registered under the Exchange Act but are not foreign private issuers, and such information may not be provided as promptly as it is provided by such companies.
The NYSE corporate governance rules require listed companies to have, among other things, a majority of independent board members and independent director oversight of executive compensation, nomination of directors and corporate governance matters. As a foreign private issuer, we are permitted to, and we do, follow home country practice in lieu of the above requirements. As long as we rely on the foreign private issuer exemption to certain of the NYSE corporate governance standards, a majority of the directors on our board of directors are not required to be independent directors, our remuneration committee is not required to be comprised entirely of independent directors and we will not be required to have a nomination committee. Therefore, our board of directors’ approach to governance may be different from that of a board of directors consisting of a majority of independent directors, and, as a result, the management oversight of our Company may be more limited than if we were subject to all of the NYSE corporate
33
governance standards. Accordingly, our shareholders may not have the same protections afforded to shareholders of companies that are subject to all NYSE corporate governance requirements.
We are obligated to maintain proper and effective internal control over financial reporting. Our internal controls may not be determined to be effective, which may adversely affect investor confidence in us and, as a result, the value of our ordinary shares.
As of December 31, 2023, we are no longer an “emerging growth company.” As a result, we are now required to comply with the independent auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act (“Section 404”), beginning with our annual report on Form 20-F for the year ended December 31, 2023. Complying with Section 404 requires a rigorous compliance program as well as adequate time and resources.
The Section 404 assessment must include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. In addition, our independent registered public accounting firm will be required to attest to the effectiveness of our internal control over financial reporting.
Additionally, in this report we have identified material weaknesses in our internal control over financial reporting, so we are unable to assert that our internal controls are effective. Accordingly we can lose investor confidence in the accuracy and completeness of our financial reports, which could have a material adverse effect on the price of our ordinary shares. We may not be able to complete the required remediation in a timely fashion.
Moreover, we are experiencing increased costs and demands on management resulting from our large accelerated filer status. To seamlessly operate through the transition from accelerated filer and emerging growth company to large accelerated filer, we have been devoting significant time and efforts to implement and comply with the additional standards, rules and regulations that now apply to us as a large accelerated filer, diverting such time from the day-to-day conduct of our business operations. Compliance with such additional requirements also will likely increase our legal, accounting and financial compliance costs. These requirements include, but are not limited to:
Due to the complexity and logistical difficulty of implementing the standards, rules and regulations that apply to a large accelerated filer, there is an increased risk that we may be found to be in non-compliance with such standards, rules and regulations. Any failure to maintain effective disclosure controls and internal control over financial reporting could materially and adversely affect our business, results of operations and financial condition.
We may lose our foreign private issuer status in the future, which could result in significant additional costs and expenses.
As discussed above, we are a foreign private issuer and, therefore, we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act. The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter, and, accordingly, the next determination will be made with respect to us on June 30, 2024. We would lose our foreign private issuer status if, for example, more than 50% of our shares were held by U.S. residents, and more than 50% of our total assets are located in the United States as of December 31, 2023. If we lose our foreign private issuer status on this date, we will be required to file with the SEC periodic reports and registration statements on U.S. domestic issuer forms beginning on January 1, 2025, which are more detailed and extensive than the forms available to a foreign private issuer. We will also have to mandatorily comply with U.S. federal proxy requirements, and our officers, directors and principal shareholders will become subject to the short-swing profit disclosure and recovery provisions of Section 16 of the Exchange Act. In addition, we will lose our ability to rely upon exemptions from certain corporate governance requirements under the listing rules of the NYSE. As a U.S. listed public company that is not a foreign private issuer, we will incur significant additional legal, accounting and other expenses that we will not incur as a foreign private issuer, and accounting, reporting and other expenses in order to maintain a listing on a U.S. securities exchange. These expenses will relate to, among other things, the obligation to present our financial information in accordance with U.S. GAAP in the future.
34
The obligations associated with being a public company require significant resources and management attention.
As a public company in the United States, we incur legal, accounting and other expenses that we did not incur prior to listing on the NYSE. We are now subject to the reporting requirements of the Exchange Act, and the Sarbanes-Oxley Act, the listing requirements of the NYSE and other applicable securities rules and regulations. Compliance with these rules and regulations increases our legal and financial compliance costs, makes some activities more difficult, time-consuming or costly and increases the demand on our systems and resources, particularly given that we no longer are an “emerging growth company.” For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to incur substantial costs to maintain the same or similar coverage, and our business, prospects, financial condition and results of operations could be materially and adversely affected. The Exchange Act requires that we file annual and current reports with respect to our business, financial condition and results of operations. The Sarbanes-Oxley Act requires, among other things, that we establish and maintain effective internal controls and procedures for financial reporting. Furthermore, the need to establish the corporate infrastructure demanded of a public company may divert management’s attention from implementing our growth strategy, which could prevent us from improving our business, financial condition and results of operations. We have made, and will continue to make, changes to our internal controls and procedures for financial reporting and accounting systems to meet our reporting obligations as a public company. However, the measures we take may not be sufficient to satisfy our obligations as a public company.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from revenue-generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us, and our business, prospects, financial condition and results of operations could be materially and adversely affected.
For as long as we were an “emerging growth company” under the JOBS Act, our independent registered public accounting firm was not required to attest to the effectiveness of our internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act. Now that we no longer are an emerging growth company, our independent registered public accounting firm is required to attest to the effectiveness of our internal control over financial reporting based on our filer status. In addition, in connection with the implementation of the necessary procedures and practices related to internal control over financial reporting, we may identify deficiencies that we may not be able to remediate in time to meet the deadline imposed by the Sarbanes-Oxley Act for compliance with the requirements of Section 404. Failure to comply with Section 404 could subject us to regulatory scrutiny and sanctions, impair our ability to raise revenue, cause investors to lose confidence in the accuracy and completeness of our financial reports and negatively affect the price of our ordinary shares.
You may experience future dilution as a result of future equity offerings.
In order to raise additional capital, we may in the future offer additional ordinary shares at prices that may not be the same as the price per share you paid. We may sell shares or other securities in any other offering at a price per share that is less than the price per share paid by existing investors, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional ordinary shares in future transactions may be higher or lower than the price per share paid by existing investors.
We may need to raise additional funds to finance our future capital needs, which may dilute the value of our outstanding shares or prevent us from growing our business.
We may need to raise additional funds to finance our existing and future capital needs. If we raise additional funds through the sale of equity securities, we may issue such additional shares at a discount to the trending price of our
35
shares, which may dilute the value of our outstanding shares. We may also decide to issue securities, including debt securities that have rights, preferences and privileges senior to our shares. Any debt financing would increase our level of indebtedness and could negatively affect our liquidity and restrict our operations. We also can provide no assurances that the funds we raise will be sufficient to finance our existing indebtedness. We may be unable to raise additional funds on terms favorable to us or at all. If financing is not available or is not available on acceptable terms, we may be unable to fund our future needs. This may prevent us from increasing our market share, capitalizing on new business opportunities or remaining competitive in our industry.
If we fail to comply with requirements relating to being a public company in the United States when obligated to do so, our business could be harmed and our ordinary shares price could decline.
We are a joint stock company with limited accounting personnel and other relevant resources with which to address our internal controls and procedures. Our registered public accounting firm previously had not performed an audit of our internal control over financial reporting during any period in accordance with the provisions of the Sarbanes-Oxley Act and it is possible that, had our registered public accounting firm performed an audit of our internal control over financial reporting in accordance with the provisions of the Sarbanes-Oxley Act, significant deficiencies and/or material weaknesses would have been identified.
The continued presence of material weaknesses and/or significant deficiencies in any future financial reporting periods could result in financial statement errors that, in turn, could lead to errors in our financial reports, delays in our financial reporting, and that could require us to restate our operating results, investors may lose confidence in the accuracy and completeness of our financial reports and the market price of our ordinary shares could be materially and adversely affected. We might also not identify one or more material weaknesses and/or significant deficiencies in our internal controls in connection with evaluating our compliance with Section 404(a) of the Sarbanes-Oxley Act. Furthermore, Section 404(b) of the Sarbanes Oxley Act (“Section 404(b)”) requires our independent registered public accounting firm to issue an annual report that addresses the effectiveness of our internal control over financial reporting. When we still had our emerging growth company status, we had opted to rely on the exemptions provided in the JOBS Act, and consequently were not required to comply with SEC rules that implement Section 404(b). Now that we are no longer an emerging growth company, our independent registered public accounting firm is required to attest to the effectiveness of our internal control over financial reporting.
In order to achieve and maintain compliance with the requirements of Section 404(a), we need to expend significant resources and provide significant management oversight. Implementing any appropriate changes to our internal controls may require specific compliance training of our directors and employees, entail substantial costs in order to modify our existing accounting systems, take a significant period of time to complete and divert management’s attention from other business concerns. These changes may not, however, be effective in maintaining the adequacy of our internal controls.
As disclosed in more detail under Item 15A, “Controls and Procedures” below, we have identified material weaknesses as of December 31, 2023 in our internal control over financial reporting. Our management has taken action to begin remediating the material weaknesses; however, certain remedial actions have only recently been undertaken, and while we expect to continue to implement our remediation plans throughout the fiscal year ending December 31, 2024, we cannot be certain as to when remediation will be fully completed. There can be no assurance that such remediation efforts will be successful, that our internal control over financial reporting will be effective as a result of these efforts or that any such future deficiencies identified may not be material weaknesses that would be required to be reported in future periods. In addition, we cannot assure you that our independent registered public accounting firm will be able to attest that such internal controls are effective.
If either we are unable to conclude that we have effective internal control over financial reporting or, at the appropriate time, our independent registered public accounting firm issue an adverse opinion on the effectiveness of our internal control over financial reporting as required by Section 404(b), investors may lose confidence in our operating results, the price of our ordinary shares could decline, and we may be subject to litigation or regulatory enforcement actions. In addition, if we are unable to meet the requirements of Section 404, we may not be able to remain listed on the NYSE.
36
ITEM 4. Information on the Company
Overview
Stevanato Group S.p.A. was incorporated on July 15, 1980, and the company has a duration set until December 31, 2100 which may be subsequently extended by the shareholders of the company. We are a joint stock company (società per azioni) incorporated in the Republic of Italy and our corporate affairs are governed by our articles of association, certain provisions of the Italian Civil Code, which we refer to as the Civil Code below, and the laws of the Republic of Italy. On July 16, 2021, our Shares are listed on the New Stock Exchange under the symbol “STVN”.
Our principal executive offices are located at Via Molinella 17, 35017 Piombino Dese – Padua, Italy and our telephone number is +39 049 931811. We have appointed Ompi of America, whose address is 41 University Drive No. 400, Newton, PA - 18940, as our agent upon whom process may be served in any action brought against us under the laws of the United States. Please see the section entitled “Enforceability of Civil Liabilities Against Foreign Persons” for more information.
For further information on important events in the development of our business, please see the section entitled “—B. Business Overview—Our Business.” For further information on our principal capital expenditures please see the section entitled “Item 5. Operating and Financial Review and Prospects—Liquidity and Capital Resources.” We have not been the subject of any public takeover offers by any third party.
The SEC maintains an internet site that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC, which can be found at http://www.sec.gov. Our internet address is www.stevanatogroup.com.
The information contained on our website is not incorporated by reference and does not form part of this annual report.
History
In 1949, Giovanni Stevanato founded Soffieria Stella, a specialty glass manufacturer, in Venice. Soffieria Stella, the precursor to Stevanato Group, operated until 1959, when Stevanato Group was established in Piombino Dese (Padua). Over the last 70 years, we have evolved from an Italian glassware manufacturer to a leading global provider of integrated solutions for the healthcare industry focused exclusively on pharmaceutical-grade products. Our growth has been driven by the internal development of new drug containment and delivery solutions, increases in capacity to support customer demand, and strategic acquisitions, enabling us to broaden our offering, our technical know-how and our international footprint.
We began our international expansion in 2005, with the acquisition of Medical Glass, a Slovakia based primary packaging manufacturing company. Subsequently, in 2007 and 2013, we acquired an Italian company, Optrel, and a Danish company, Innoscan. Both specialize in the production of inspection machines. These acquisitions marked our entry into the technology and equipment manufacturing business. In 2016, we pursued further expansion of our offering through the acquisition of: (i) Balda, a company specialized in developing and manufacturing plastic diagnostic consumables, drug delivery systems and medical components; (ii) SVM, a company specialized in the production of high-technology machines and systems for assembly, packaging and serialization of pharmaceutical products; and (iii) Medirio, a start-up developing patents and other intellectual property for the wearable injectors business.
In 2024, we will complete the consolidation of our brand architecture and market our products, processes and services under the Stevanato Group Brand. Previously we marketed our products, processes and services under several different names.
In parallel with our acquisition strategy, we regularly review our operations in the context of our organic growth plan. As a result of these ongoing assessments, we have expanded our offering through new departments, new laboratories, new offices and new plants. We opened two new buildings in Piombino Dese (Italy) in 2019 and 2023
37
to increase our syringes production capacity and since 2008, we have opened three greenfield sites in (i) Monterrey, Mexico in 2008; (ii) Zhangjiagang, China in 2012; and (iii) Sete Lagoas, Brazil in 2017.
In October of 2021 we announced the construction of a new EZ-Fill® hub in Fishers, Indiana. The plant launched customer validation activities, and we expect to begin commercial operations in 2024.
In December 2021, we acquired an existing facility in Zhangjiagang, China for a new plant where we expect to increase capacity and production for our pre-sterilized EZ-fill® syringes and vials, as well as visual inspection machines and glass forming lines. In 2023, we decided to delay the timing of our investment in China in order to focus efforts and resources on our manufacturing facilities in the U.S. and Italy where demand has outpaced our initial expectations.
In May of 2022, we acquired a brownfield facility in Latina (Italy) for the production of EZ-fill® syringes and cartridges. Validation activities were launched in 2023, and the facility began commercial production in the fourth quarter of 2023.
On November 8, 2023, we acquired all of the business operations of Perugini S.r.l., an Italian company specialized in the manufacturing of consumables and mechanical components for industrial machines for a total consideration of approximately €6.4 million. The acquisition of Perugini will support the Group's efforts in the ongoing integration of critical technologies and processes into the Group’s production process.
We are a leading global provider of drug containment, drug delivery and diagnostic solutions as well as engineering solutions to the pharmaceutical, biotechnology and life sciences industries.
We deliver an integrated, end-to-end portfolio of products, processes and services that address customer needs across the entire drug life cycle from development to clinical and commercial stages. Our core capabilities in scientific research and development, our commitment to technical innovation and our engineering excellence are central to our ability to offer value added solutions to our clients.
We have secured a leadership position within the drug development and delivery value chain through our investment in research and development and the expansion of our global footprint and capabilities. Over our 70-year history, we have earned a leading reputation for high quality and reliability that has enabled us to become a partner of choice for more than 700 companies globally, including all of the top 25 pharmaceutical companies, and eight of the top ten in-vitro diagnostic companies, as measured by 2022 revenue, according to data collected by Pharmacircle and public companies’ information. We also serve seven of the top ten biotechnology companies (by market capitalization listed in the Nasdaq Biotechnology Index), and over 100 biotechnology customers in total.
Our priority is to provide flexible solutions that preserve the integrity of pharmaceutical products and enable our customers to deliver safe and effective treatments to patients while reducing time to market, total cost of ownership (i.e., logistics, drug product waste, storage and personnel costs) and supply chain risk. We achieve this by developing our products in close collaboration with our customers, leveraging our scientific research capabilities, technical expertise and engineering and manufacturing excellence to meet their quality requirements.
Our solutions are highly integrated with the development, production and commercialization processes of our customers. In addition to manufacturing drug containment and delivery solutions, we provide a full set of services across all stages of drug development, from pre-clinical to clinical and commercialization. We also engineer machinery and equipment for the production of drug containment and delivery systems that can be integrated into both our customers’ and our own manufacturing processes. Our involvement at each stage of a drug’s life cycle, together with the breadth of our offering, enables us to serve as a one-stop-shop for our customers, which we believe represents a significant competitive advantage.
The chart below illustrates our mission-critical presence across the pharmaceutical value chain.
38

We operate across the healthcare industry and serve some of its fastest growing segments, including biologics (such as GLP1s, monoclonal antibodies, and mRNA applications), biosimilars, vaccines and molecular diagnostics. We are closely integrated in the drug production and delivery supply chain, and we are well-positioned to benefit from secular trends within our target industries, such as increases in demand resulting from pharmaceutical innovation, acceleration and expansion of vaccination programs, growth of biologics/biosimilars, self-administration of medicines, aging demographics, increasing complexities in health conditions and co-morbidities, and increasing quality standards and regulation.
We estimate that our total addressable market, based on our current offering, exceeds $15 billion, in terms of revenue generated by all market participants in 2023, and consists primarily of biopharmaceutical injectables and in-vitro diagnostic products. The addressable market estimation is based on revenue from all market players in 2023; if revenue from 2023 is not available, revenue for 2022 is used applying market growth rate from third parties such as IQVIA, Roots Analysis, Markets and Markets Research, and Alira Health. Within each of these markets, we operate in some of the fastest growing segments, including pre-fillable syringes, vials and cartridges, drug delivery systems, molecular diagnostics and assembly equipment. We believe there are opportunities to further expand our addressable markets, including by targeting (i) complementary containment solutions, (ii) additional delivery systems, (iii) complementary engineering solutions, and (iv) aftersales support and other services.
We operate our business in two segments:
The figure below provides a breakdown of our segments, as well as the business lines included within each segment.
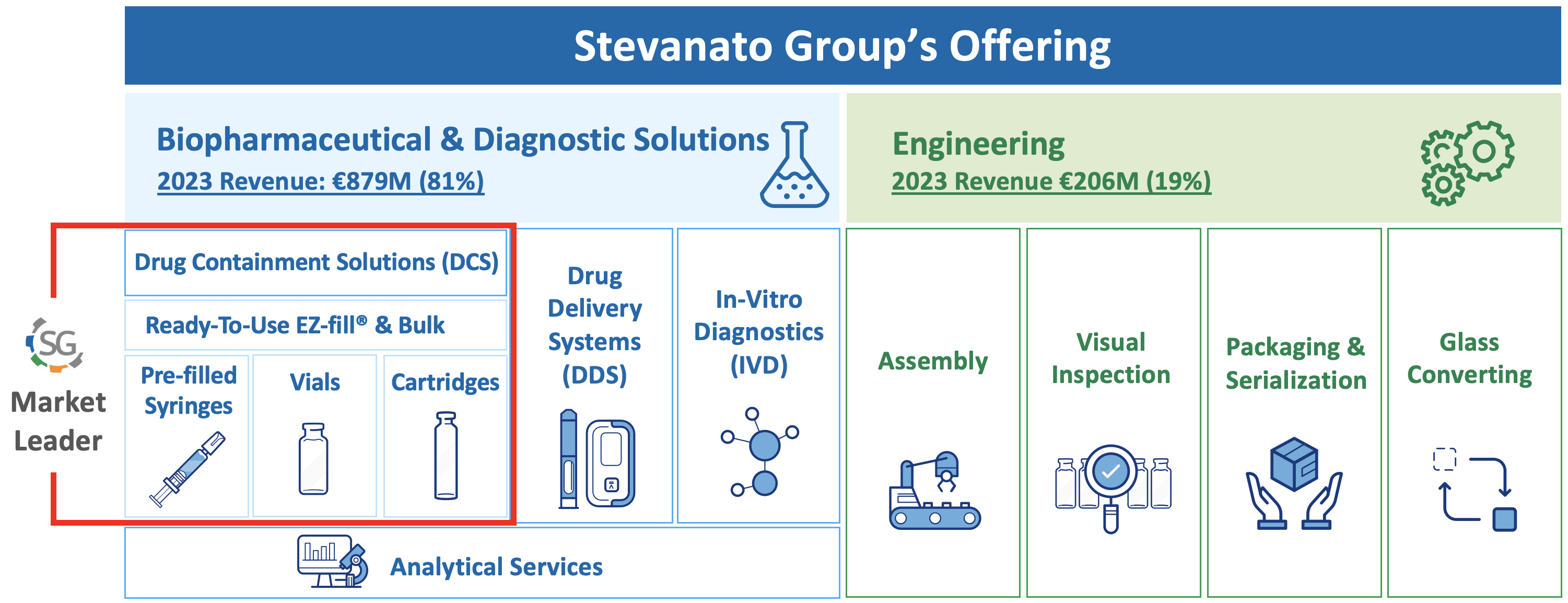
39
In 2023, we generated 81% of total revenue from our Biopharmaceutical and Diagnostic Solutions segment and 19% from our Engineering segment. The principal markets in which we operate and compete include the EEA, the U.K., Asia (with a focus on China) and the Americas (with a focus on Brazil and Mexico in South America, and the U.S. in North America). Our two main business segments (Biopharmaceutical and Diagnostic Solutions, and Engineering), combined with our global footprint, allow us to sell products and provide services in over 70 countries worldwide which we achieve mostly through business‑to‑business marketing channels and selected distributors. Please see the section entitled “Item 5. Operating and Financial Review and Prospects” for a more detailed description of our revenue generating activities.
We refer to premium products in the Biopharmaceutical and Diagnostic Solutions segment as our high-value solutions. High-value solutions are products and services for which we hold intellectual property rights or have strong proprietary know-how, and that are characterized by technological and process complexity and high performance. Our high-value solutions deliver significant benefits to customers including, faster time-to-market, lower total cost of ownership and higher flexibility. Among our key high-value solutions is our EZ- Fill® line of ready-to-fill injectable products, which can be customized to clients’ needs. For additional information on EZ-Fill® see “Business—Business Segments—Biopharmaceutical and Diagnostic Solutions— Drug Containment Solutions (DCS)”.
We have nine production plants for manufacturing and assembly of bio-pharma and healthcare products (in Italy, Germany, Slovakia, Brazil, Mexico, China, and the United States), six plants for the production of machinery and equipment (in Italy and Denmark), two sites for analytical services (in Italy and the United States) and two commercial offices (in Japan and the United States).
We are expanding our production in Piombino Dese (Italy), Latina (Italy), and Fishers (Indiana, the U.S). In 2023 we decided to delay the timing of our investment in China to focus our resources and efforts in completing our manufacturing expansion in the U.S. and Italy. Our global footprint allows us to sell products and provide services in more than 70 countries worldwide.
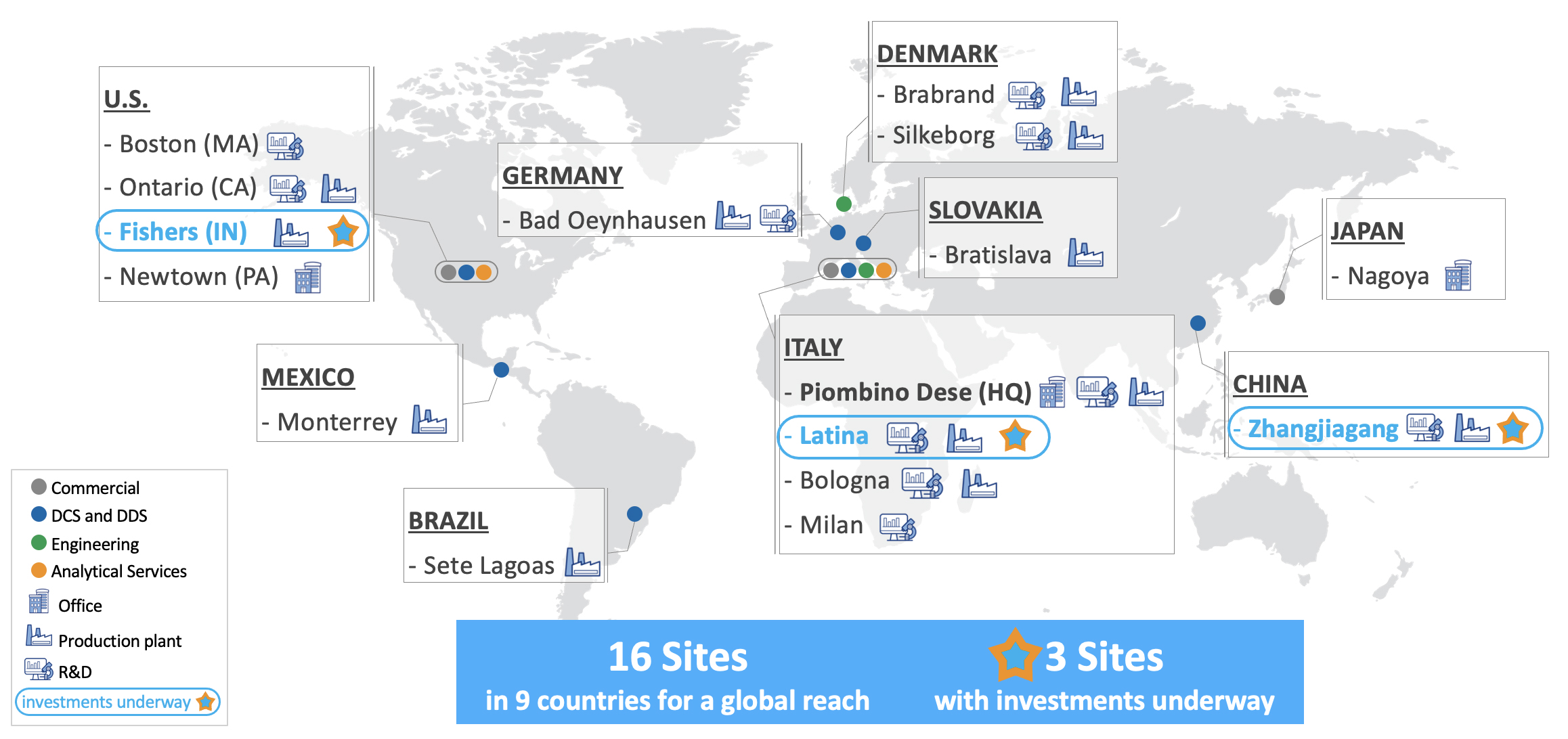
During COVID-19, we increased production capacity to support the rapid response to COVID-19 and we have been providing: (i) glass vials and syringes to approximately 90% of the currently marketed vaccine programs, according to our estimates based on public information (WHO, EMA, FDA); and (ii) plastic diagnostic consumables for the detection and diagnosis of COVID19. In 2021 and 2022, COVID-19 generated increased demand for our products and services. In 2023, demand for COVID-19 related products decreased as the pandemic waned, but we backfilled the revenue decreases from COVID-19 with new projects.
There remains some uncertainty around the magnitude of the long term demand for COVID-19-related products, but we anticipate that COVID-19 contributions will likely normalize in 2024 and transition into our ongoing vaccination business.
40
Our Industry and Growing End Market
We are a key partner to leading companies in the pharmaceutical, biotechnology and life sciences industries, serving as one of the preeminent providers of drug containment, drug delivery and diagnostic solutions as well as engineering systems to these end markets.
We estimate that our total addressable market, based on our current portfolio of products and services comprising drug containment solutions, drug delivery systems, IVD solutions, and engineering, exceeds $15 billion in 2023. Our addressable market estimation is based on revenue generated by all market players in 2023; if revenue from 2023 is not available, revenue for 2022 is used applying market growth rate from third parties such as IQVIA, Roots Analysis, Markets and Markets Research, and Alira Health.
Drug containment solutions and drug delivery systems represent mission-critical components of the pharmaceutical and biotechnology value chain for injectable drugs, which are produced for the treatment of a wide range of diseases from diabetes to cancer and other chronic conditions. Due to our competitive standing, we believe that we are well positioned to capitalize on several major demographic and technological trends generating growth in the global healthcare markets, including:
We categorize our addressable market by direct markets and end markets. Our direct markets are comprised of products and product categories in which we directly participate, such as drug containment solutions. Our end markets include the broader sectors from which we see demand for our products and services, such as vaccines and biologics.
Direct Markets:
The below chart illustrates the direct markets we serve.
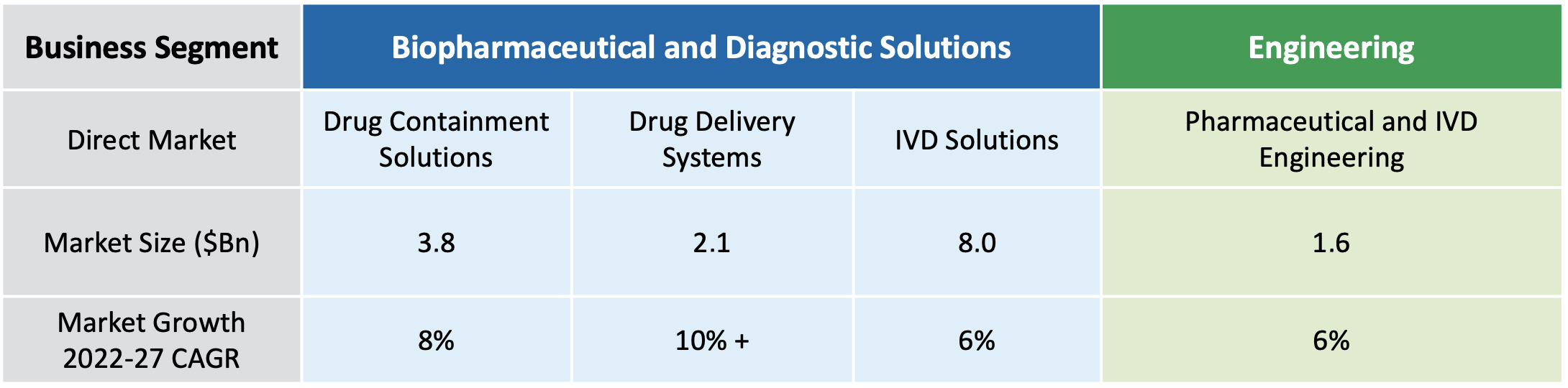
Drug Containment Solutions (“DCS”)
The DCS market includes the markets for pre-fillable syringes (“PFS”), standardized and ready-to-use vials, cartridges and ampoules. Based on data collected by IQVIA and internal elaborations, we estimate the total addressable market of DCS solutions to be approximately $ 3.8 billion as of 2022, including the temporary impact of
41
COVID-19 programs. Excluding the impact of COVID-19, we expect the market to grow at a Compounded Annual Growth Rate (“CAGR”) of approximately 7% to 9% through 2027. Growth in the DCS market is driven by the growing number of injectable drug launches – both innovator and generics/biosimilar – and increased treatment access globally which are expected to generate continued demand for pre-fillable syringes, vials, and cartridges. Customers in this market increasingly seek out “ready-to-use” products which include pre-sterilized offerings and ready-to-use packaging (PFS, vials, cartridges and ampoules) that provide pharmaceutical customers with higher flexibility, lower total cost of ownership and reduced time to market. In addition to these underlying drivers of our core business, the impact of COVID-19 and international vaccination programs are expected to produce further volume growth in pre-fillable syringes and vials. We are well positioned to capitalize on the highest-growth segments of the DCS market, with pre-fillable syringe and vial sub-segments estimated to grow at a CAGR of approximately 10% and 7% to 8% towards 2027 respectively, excluding COVID-19.
Drug Delivery Systems (“DDS”)
Our addressable market in DDS, including both Contract Manufacturing Organizations and Contract Development and Manufacturing Organizations, consists of pen-injectors, dry powder inhalers, auto injectors, and non-insulin wearable devices. Based on data collected by IQVIA, Roots Analysis, Markets and Markets Research and internal elaborations, we estimate the total addressable market for DDS, including proprietary and contract development manufacturing services, to be approximately $ 2.1 billion as of 2022, and the market is expected to grow at a CAGR of above 10% through 2027. Growth in the DDS market is driven by increased demand for pen-injectors, autoinjectors, wearable devices and dry powder inhalers for large, established drug classes such as insulin, as well generics and biosimilars. The increasing prevalence of diabetes and asthma, as well as expanded access to treatments that improve patient care and flexibility support continued growth in these markets.
In-Vitro Diagnostic (“IVD”) Solutions
The IVD solutions market consists of diagnostic devices and consumables for point-of-lab and point-of-care use. Based on data collected by Alira Health, we estimate the total addressable market for IVD solutions to be approximately $ 8.0 billion as of 2022, and the market is expected to grow at a CAGR of approximately 5% to 6% through 2027. Our IVD solutions are mainly utilized in molecular diagnostics, immunoassays and clinical chemistry development and manufacturing. Molecular diagnostics growth is primarily driven by technology advancements, with increasing need for sensitivity and specificity in testing. Immunoassay growth is driven by the increasing impact of infectious disease and oncology testing. Finally, clinical chemistry consists of testing conducted by established large market participants. Both growing and established companies increasingly utilize cost-efficient manufacturing partners with expertise in design and manufacturing. We increasingly target the market for molecular diagnostics within IVD solutions, which we estimate to be growing faster than the broader market for IVD solutions at a CAGR of approximately 9% to 11% through 2027, based on Alira Health analysis.
Pharmaceutical & IVD Engineering ("Engineering")
Our pharmaceutical and IVD engineering addressable market consists of assembly, visual inspection, packaging and serialization and glass converting machines. According to Alira Health and Markets and Markets Research Pvt Ltd. and Acumen Research & Consulting analysis, the total addressable market of pharmaceutical and IVD engineering was approximately $ 1.6 billion as of 2022, and the market is expected to grow at a CAGR of approximately 6% through 2027. This market requires critical engineering know-how developed over numerous years as well as regulatory approvals to market machinery. We believe there will be increasing regulatory scrutiny, growing trends toward more complex manufacturing systems, and a rising need for digitalization and automation of manufacturing. The market is expected to experience continued growth as the industry shifts towards enhanced service offerings and aftersales support. Consequently, aftersales services, including spare parts provisioning, machinery upgrades, periodic maintenance and warranty extensions, represent a critical portion of our growth derived from this segment. Within the pharmaceutical and IVD engineering market, we are increasingly targeting the market for assembly equipment, which we estimate to be growing at a CAGR of approximately 6% to 7% through 2027, based on Alira Health analysis.
42
Key End Markets Segment:
The key end market segments that we serve include biopharmaceutical injectables, which represent the majority of our business, as well as the rapidly growing in-vitro diagnostic sector.
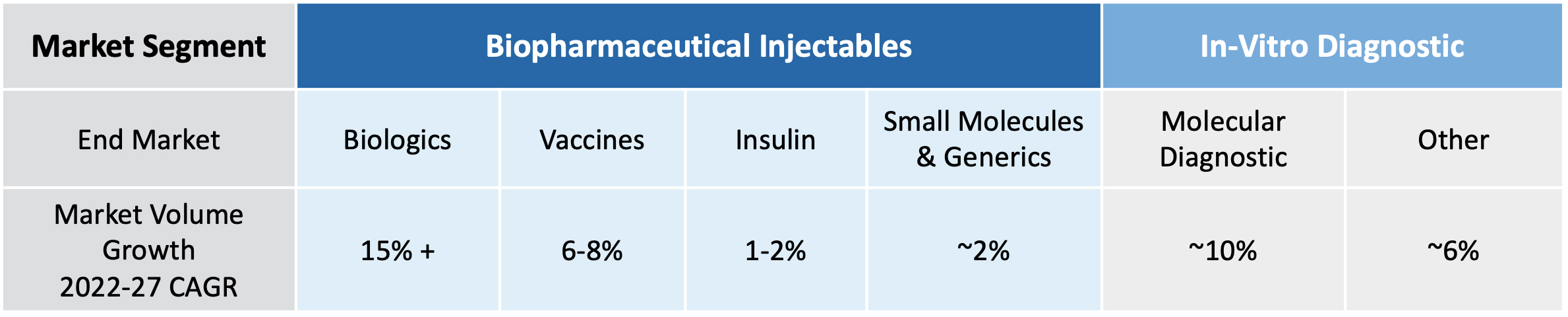
Biopharmaceutical Injectables
The biopharmaceutical injectables end market comprises multiple distinct injectable drug categories such as biologics, vaccines, small molecules and generics, and insulin. According to data collected by IQVIA, the market for biopharmaceutical injectables – in terms of volume of primary container standard units – is expected to grow at a CAGR of approximately 2% to 4% through 2027, outpacing growth in topical and oral administration.
We increasingly serve some of the fastest growing segments within biopharmaceutical injectables, such as:
We also serve more mature and established markets such as:
In-Vitro Diagnostic
In-vitro diagnostic is an important and growing end-market where we participate with a focus on molecular diagnostics, point-of-care diagnostics and, increasingly, infectious diseases and oncology. With an increasing number of diseases to which molecular diagnostic technology and rapid advances in genomics can be applied, the molecular diagnostics end market is expected to continue to experience high growth. Additionally, the impact of COVID-19 has highlighted the growing importance of advanced diagnostics capabilities, contributing to further growth in this market.
43
Based on market data from Alira Health, Transparency Market Research, and Data Horizon Research, the in-vitro diagnostic end market is expected to grow at a CAGR of approximately 5% to 7% through 2027, with molecular diagnostics showing a higher growth rate of approximately 10%.
The following chart provides a revenue breakdown by end market for the Biopharmaceutical and Diagnostics Solutions Segment:
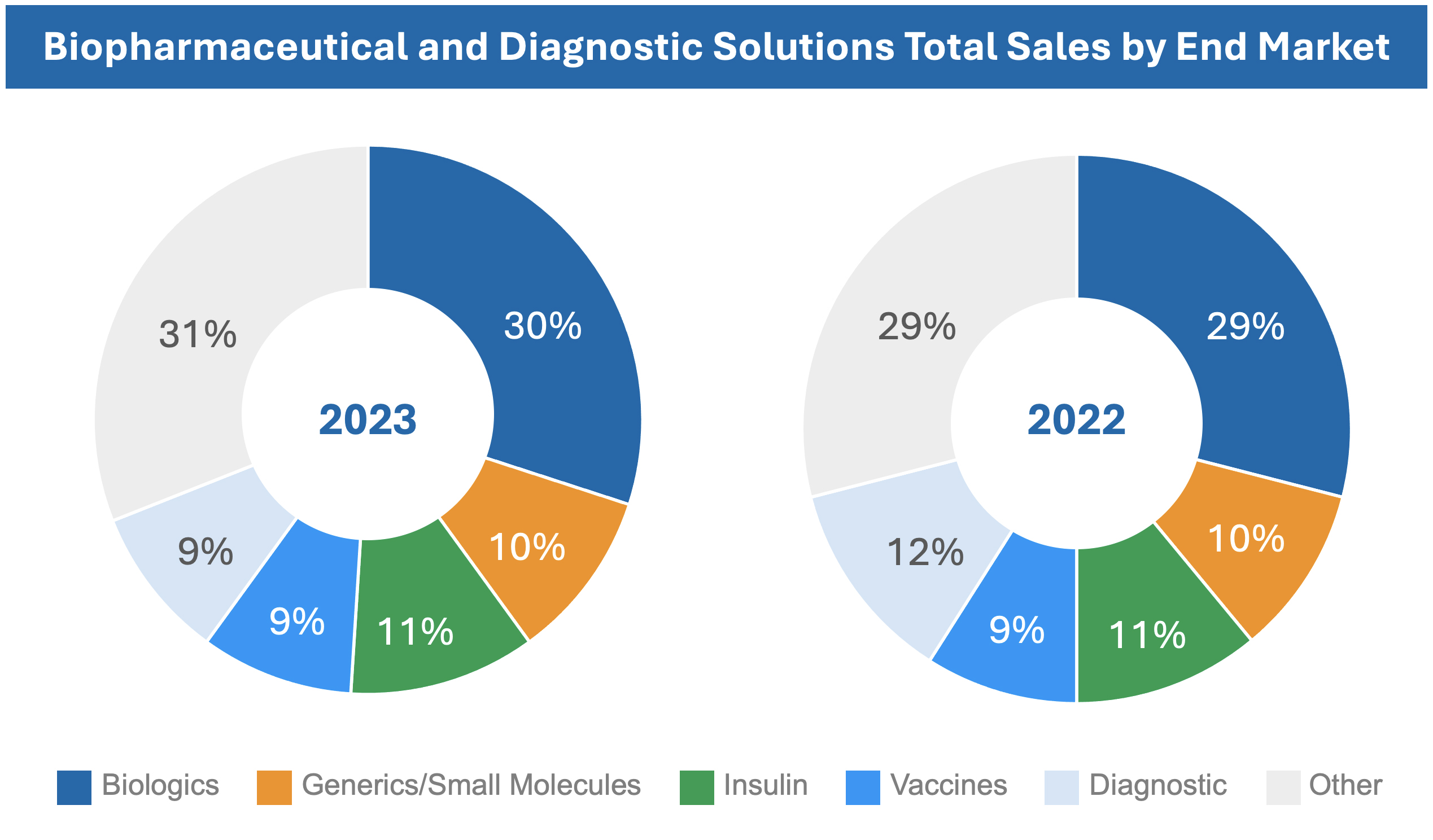
Our Competitive Strengths
We have secured a leadership position as a critical solutions provider in the drug development and delivery value chain. Our integrated offering and track record of operational excellence has made us a partner of choice to the pharmaceutical, biotechnology and life sciences industries.
We benefit from several competitive advantages that we believe will allow us to continue to deliver for customers and remain at the forefront of the markets in which we operate. The following are our key competitive strengths:
Leading global provider of mission-critical drug containment, delivery and diagnostic solutions for the pharmaceutical, biotechnology and life sciences industries and a trusted partner for the production of world-class manufacturing equipment to support customers worldwide
We are a recognized leader in providing mission-critical drug containment, delivery and diagnostic solutions to the pharmaceutical, biotechnology and life sciences industries. We operate on a global scale, offering our products, processes and services in more than 70 countries. We serve a large and diversified customer base, including many of the world’s largest pharmaceutical, biotechnology and diagnostics companies, contract manufacturers and producers of glass packaging. Our customer base comprises more than 700 companies globally, including all of the top 25 pharmaceutical companies, and eight of the top ten in-vitro diagnostic companies, as measured by 2022 revenue, according to data collected by Pharmacircle and public companies’ information. We also serve seven of the top ten biotechnology companies by market capitalization in the Nasdaq Biotechnology Index and over 100 biotechnology customers in total.
As a partner of choice to the pharmaceutical and biotechnology industries, our solutions have been widely adopted, giving us a leading position in several high growth segments of the pharmaceutical and biotechnology injectables market, including in biologics, biosimilars and vaccines. Within each of these markets, we operate in some of the fastest growing segments, where, based on available market data, we believe we are a global top three player by revenues, including number two in pre-fillable syringes, number one in pre-sterilized EZ-Fill®, ready-to-use vials and number one in pen cartridges. Our decades-long expertise in manufacturing equipment for customers through our Engineering Segment differentiate us from our competitors, allowing us to offer end-to-end services.
44
Integrated end-to-end platform spans the drug lifecycle, from design and development through commercialization
We offer solutions to our clients at each stage of the drug development process, from research and development, through clinical trials and commercialization. Our fully integrated, end-to-end value proposition allows us to reduce lead times, total cost of ownership (i.e., logistics, drug product waste, storage and personnel costs) and supply chain risk for our customers, while improving the reliability and safety of drug products.
The breadth of our integrated capabilities differentiates us from our competitors as we believe we are the only player in the industry to be active in both the drug containment, delivery and diagnostic solutions, as well as the engineering segments. Our comprehensive product portfolio makes us an attractive partner to both small, emerging businesses, which may look to outsource a portion of their manufacturing process, as well as to mature, commercial stage drug development organizations, that require complex engineering solutions that can be integrated into their own production processes. By partnering with customers in the early development phase, we are in a prime position to play a key role as they add products to their pipelines and seek more advanced technical solutions. Our ability to seamlessly integrate our drug containment and delivery solutions with our engineering capabilities allows us to deliver significant value to our customers by reducing lead times, total cost of ownership (i.e., logistics, drug product waste, storage and personnel costs) and supply chain risk, while improving the reliability and safety of drug products and reducing the time necessary to market them.
A common operating model in all our manufacturing facilities provides a single, consistent quality standard worldwide
Our manufacturing approach is based on the relentless pursuit of maximum efficiency and highest quality. Our manufacturing methods and processes are standardized across all of our production facilities, utilizing the same technology and implementing common quality controls. This allows us to provide uniform products, processes and services, both in terms of quality and time to market, to all of our customers from each of our manufacturing locations worldwide. It also gives us the flexibility, where needed, to distribute and balance production across our facilities reducing waste and maximizing our efficiency as a group. Many of our customers depend on a diversified supply chain and access our products and services from multiple facilities globally.
As a result of our commitment to manufacturing excellence and the breadth of our footprint, our customers view us as a functional extension of their operations. We are subject to rigorous audits by certification bodies and our customers, who perform more than 150 audits a year on our manufacturing facilities. Further, given our reputation for reliability and our ability to establish new manufacturing facilities with the same standards as our existing ones anywhere in the world, our customers often coordinate with us to support their geographic expansion strategy by building out greenfield manufacturing facilities. This, in turn, provides us access to customers and allows us to further secure our long- term relationships with them.
45
Highly collaborative approach resulting in deeper strategic partnerships with clients and leading to high customer retention
We approach every customer relationship with the goal of partnering and adding value over a long-term horizon, leveraging our technical expertise and our ability to collect analytical data to fully understand our customers’ objectives, needs and limitations. Drug containment and delivery solutions are often borne out of years of collective effort with customers to develop the optimal manner of containing and delivering a drug product to patients. The customized solutions we provide vary depending on the characteristics and chemical composition of the pharmaceutical products, logistical needs (for example, ease of transport and shelf- life), patient community to which the drug product is primarily addressed (including, potentially, its geographic location) and specific regulatory requirements. The containment and delivery solutions that we provide are an integral part of the drug product itself and are included as part of the regulatory filings required to approve drug product marketing and commercialization. Providing high-quality products with specificity, sensitivity and consistency, coupled with extensive product validation data are fundamental drivers of customer loyalty. The quality and dependability of our drug containment and delivery solutions are critical to obtaining commercialization and marketing approval from regulatory agencies. As a result, it is often the case that drug product containment and delivery arrangements cannot be changed without amending the regulatory filing with the relevant agency. High switching costs and significant time delays are meaningful deterrents to a change in suppliers, which reinforces customer loyalty and strengthens customer retention. Extensive scientific and engineering capabilities enable continuous innovation of proprietary products and processes.
During our 70-year history, we have differentiated our company from others by making significant ongoing investments in research and development to build our scientific, technical and engineering capabilities. We believe that customers rely on us because of our technical expertise, as well as our ability to design high quality containment format with the best possible processes to meet their needs and the specifications required to effectively contain and deliver their drugs. As the needs of our clients evolve, we drive innovation within our proprietary products and processes to develop specialized or customized solutions. As a result of our investments in internal engineering capabilities, we own the most critical processes behind the products we manufacture and can respond faster to customer needs for new or customized products. Our engineering capabilities also allow us to scale up our production rapidly, where required, thereby reducing lead times for commercialization of drugs. Our research and development team comprises more than 150 highly skilled and specialized employees based in our facilities in Italy (Piombino Dese and Milan), Germany (Bad Oeynhausen) and the U.S. (Boston). We have an active pipeline of more than 70 ongoing R&D projects. Our targeted investment in innovative products and technologies allows us to capture incremental pipeline opportunities and drive attractive growth, while delivering on our firm-wide commitment to provide the highest quality to customers.
Experienced board and executive leadership team with proven track record of excellence
We are led by an experienced and highly motivated Board of Directors and executive leadership team with a proven track record of operational excellence. Our leadership team has consistently achieved results by responding to market developments and by capitalizing on opportunities for organic and inorganic growth. While our founding family continues to support our success and future growth, we have, over the last several years, added to our Board and executive team a number of professionals with decades of experience in the pharmaceuticals and life sciences sectors, with a particular emphasis in drug containment and packaging, delivery and diagnostics industries from all over the world. We believe that this has contributed greatly to our strategy building and execution capabilities by allowing us to gain a broader and more nuanced understanding of the markets in which we operate, strengthening our ability to anticipate market trends and enhancing our competitive advantages. Our strong corporate culture allows us to continuously expand these perspectives by adding diverse talent with deep knowledge and broad experience to our team.
Our Growth Strategy
We believe that the breadth and quality of our products and services offerings, our technical understanding of the drug-material interface, our innovative engineering and manufacturing excellence position us well to serve our global pharmaceutical, biotechnology and life sciences customers. We believe that our integrated solutions resonate with our customers, and we work with customers to move them up the product value chain as they bring new treatments to market that require more complex, integrated solutions. We believe that we will continue to benefit from favorable macro trends such as aging demographics, with more complex health needs; pharmaceutical innovation and the
46
growing demand for biologics and biosimilars; increasing quality to meet market expectations; and an increasing trend towards outsourcing non-core functions by our customers which helps to reduce the total cost of ownership for a treatment, increase flexibility and reduce the time it takes to get a new treatment to market.
We focus on our customer needs and the market trends described in the “Our Industry and Growing End Market” section and tailor our growth strategy to such needs and trends.
Our growth strategy currently focuses on the following areas:
Expand our global market position in primary containment solutions and grow our mix of high value solutions
We pursue attractive, organic growth trends in our core primary container business by investing in additional capacity to meet the growing demands of the expanding pharmaceutical, biotechnology and vaccine markets. As more complex treatments are developed, customers are transitioning up the product value chain to high-value solutions which offer superior quality and performance. In response to customer demand trends, our current geographic and capacity expansion plans are mainly focused on investments in our high value solutions product portfolio. We rely on a unique set of proprietary manufacturing processes to drive product innovations in our primary container business that benefit our customers. For example, EZ-Fill® containers enable our customers to reduce the time necessary to market a drug, while lowering their overall total cost of ownership. We reduce our supply chain risk by expanding our development capabilities and manufacturing capacity in North America, Europe and Asia to better serve our customers in our key end markets and meet the demand for high-value solutions. We believe we will be able to continue developing our offering, particularly in biologics, to generate above-market growth and capture market share across our business segments. Our planned expansion in Italy, the U.S., and China also offers our customer base faster response time and supply chain redundancy, reducing risk for just in time manufacturing.
Leverage leadership in primary containment and engineering solutions to build market position in drug delivery systems
We see a significant opportunity in the fast-paced evolution of drug delivery systems, especially in connection with biologic based therapies administered by injection. We believe that we can leverage this favorable trend in the drug delivery systems market by investing in and strengthening the integration of our drug containment solutions, engineering expertise – specifically, assembly and packaging technologies –and drug delivery capabilities in an effort to have the most compelling value proposition for our customers. In particular, we believe that by increasing the integration of our offering we can attract business from emerging biotechnology customers who have an increasing inclination to outsource the non-core phases of their development and manufacturing processes. We intend to strengthen our design and development capabilities to secure high-value contract development and manufacturing programs for drug delivery devices, also leveraging our positive track-record in the space and our ability to develop and acquire proprietary systems. We believe we offer an efficient value proposition through our fully integrated end-to-end product solutions that offer customers the ability to streamline their DDS manufacturing and assembly through a single partner.
Accelerate market penetration in attractive life sciences segments
Through focused marketing and business development activities, we are striving to accelerate our market penetration in high-value, fast-growing life sciences segments, such as molecular and point-of-care diagnostics. With the increasing tendency of life sciences customers to outsource innovative design, development and assembly of specialized in-vitro diagnostic solutions, we believe that we can leverage our integrated capabilities and our ongoing efforts in design and development of such solutions to secure high-value projects from inception, therefore entering the market at an even earlier stage and capitalizing on new opportunities.
Increase our investments in research & development to address unmet market needs
Through continued investment in our R&D programs, we see opportunities to leverage our scientific and technological capabilities to drive revenue and margin growth through processes that improve the quality and sustainability of our existing products. These investments are targeted at maintaining the stability, potency and purity of our customers’ products prior to administration. New therapies for diabetes, cancer and auto-immune diseases are based on large, complex molecules that are extremely sensitive to their storage environment. In many cases, our
47
customers’ finished product formulations are viscous and require drug delivery devices for administration to patients. Our products, such as EZ Fill®, reduce our customers drug containment risks, such as the ones mentioned above. We also see growing interest within our customer base in systems that detect tampering, anti-counterfeiting, inventory track and trace capability, and in the case of devices, smart systems that allow patient data capture.
Easy-to-use, accurate, reliable self-injection systems for complex pharmaceutical and biotechnology products represent a particularly attractive market opportunity. We have built a portfolio of devices for this market that can be used off the shelf or customized to the specific needs of the customer.
We also see a growing market need for innovative containment and delivery systems for advanced cell and gene therapies. Effective solutions for these products will require innovative materials and coatings, system design and stability and compatibility testing, all of which are areas of strength for our development teams.
Build on our expertise in manufacturing, assembly and inspection systems for primary containers and complex, multi-component systems
Our market leading expertise in the design and manufacturing of glass converting systems for drug containment offers the opportunity to grow in complex, multi-component systems. Working closely with our customers, we can offer custom designed systems complete with artificial intelligence-enabled vision inspection technology to assure the highest quality products. The enhanced scalability and flexibility of our assembly and packaging solutions are well suited to match emerging biotechnology customers’ requirements such as smaller production batches with higher variability in dosage formats. We see future opportunities to apply these solutions to manufacturing multi-component devices for in vitro diagnostics, including point-of-care and self-injection devices for pharmaceutical and biotechnology customers.
Leverage our scientific and engineering capabilities across the drug development timeline
We have created an integrated, end-to-end, flexible portfolio of products, processes and services that offers early entry in the drug development stages. We collaborate with customers from the preclinical phase through Phase III testing, regulatory filing and eventual commercialization. We believe that our ability to assist from the early stages of preclinical development is an important element in pursuing the opportunity to gain new customers.
Such close collaboration allows us to leverage our scientific and engineering capabilities to strengthen and expand our business relationships. By assisting customers through their production processes, we gain the visibility and knowledge that, combined with our skills and capabilities, allow us to anticipate their emerging needs and intercept new demands. We address these needs by continuing to expand our product offering and making new solutions available. Through close collaboration with our customers, we gain invaluable insight into system requirements and industry trends and challenges, which we re-deploy for our future development projects, or to secure new business. For instance, we intend to pursue new opportunities driven by the trend of biotechnology companies toward outsourcing non-core activities of their business.
Leverage our global geographical presence as a platform to increase our penetration in the North American and Asia Pacific regions
The North American and APAC regions are two of the fastest growing markets and represent significant growth opportunities for our company. Both markets have well established research and manufacturing capabilities for biologic therapies covering both innovator and biosimilar products. We have a small but rapidly growing position in both regions, where we believe we can accelerate our recent growth by further expanding our manufacturing footprint. By providing locally sourced products, we can deliver supply chain security, just in time delivery, and reliable sourcing in terms of surge capacity to both existing and new customers. For example, our new plant in Indiana (U.S.) will represent a strategic location for us in proximity to key emerging biopharma players, enabling us to access an attractive biotech and vaccine market. We believe that we are well-positioned to expand our footprint and market share in the North American and Asia Pacific regions. In an effort to grant access to treatments and vaccines to a higher portion of their population and, therefore, improve their quality of life, APAC countries are showing a consistently growing demand for biologics and cell and gene therapy solutions, as well as strong inclination towards investment in biosimilars. We believe that our global footprint will allow us to take advantage of these favorable growth trends. We intend to further invest in the North American and APAC regions to increase our market penetration in these regions
48
across the business segments in which we operate. In March 2023, we elected to slow down our investment in China in order to focus on the execution of our new manufacturing facilities in the United States and Italy.
Selectively pursue acquisitions and technology partnerships to augment and expand our product and service portfolio
Our acquisition strategy is opportunistic and focused on adding complementary or adjacent offerings. We have a proven track record of successfully identifying, completing and integrating newly acquired complementary businesses and technologies. Our extensive knowledge of the competitive landscape and deep understanding of the evolving needs of our customers and end markets enable us to identify actionable opportunities to expand our portfolio. We employ a disciplined process to evaluate the strategic fit and financial prospects of acquisitions using a well-established set of criteria.
Business Segments
Our business operations are organized into two reporting segments: (i) Biopharmaceutical and Diagnostic Solutions, which includes all the products and services developed and provided for the containment and delivery of pharmaceutical and biotechnology drugs and reagents, as well as the production of diagnostic consumables, and (ii) Engineering, which includes all the equipment and technologies developed and provided to support the end-to-end pharmaceutical, biotechnology and life sciences manufacturing processes (visual inspection, assembly, packaging and serialization and glass converting). In 2023, we generated 81% of total revenue from our Biopharmaceutical and Diagnostic Solutions segment and 19% from our Engineering segment.
Biopharmaceutical and Diagnostic Solutions Segment
Through our Biopharmaceutical and Diagnostic Solutions segment, we offer a wide range of development and manufacturing solutions to our pharmaceutical, biotechnology and life sciences customers. This segment comprises drug containment solutions (DCS), in-vitro diagnostic (IVD) solutions and drug delivery systems (DDS). We also provide analytical services and regulatory support exclusively to our customers, as ancillary services to the supply of containment solutions.
The Biopharmaceutical and Diagnostics Solutions segment includes our high-value solutions. These solutions are products, processes and services for which we hold intellectual property rights and have proprietary know-how and are characterized by particular complexity and high performance. Our high-value solutions represent a cross-section of our portfolio, including glass and polymer drug containment solutions such as NEXA®, NEXA FlexTM, LDP, ALBA® and a significant proportion of our EZ-Fill® line, as well as other drug delivery devices, molecular diagnostic solutions and analytical services.
Due to the technical complexity of our high-value solutions, and the significant value these generate for our customers, we enjoy premium pricing on these products, services and processes. Over time we have expanded our offering of high-value solutions, enabling us to drive significant growth from this category. Over the last five years, revenue from our high-value solutions has steadily grown and represented 34% of our total revenue for the year ended December 31, 2023.
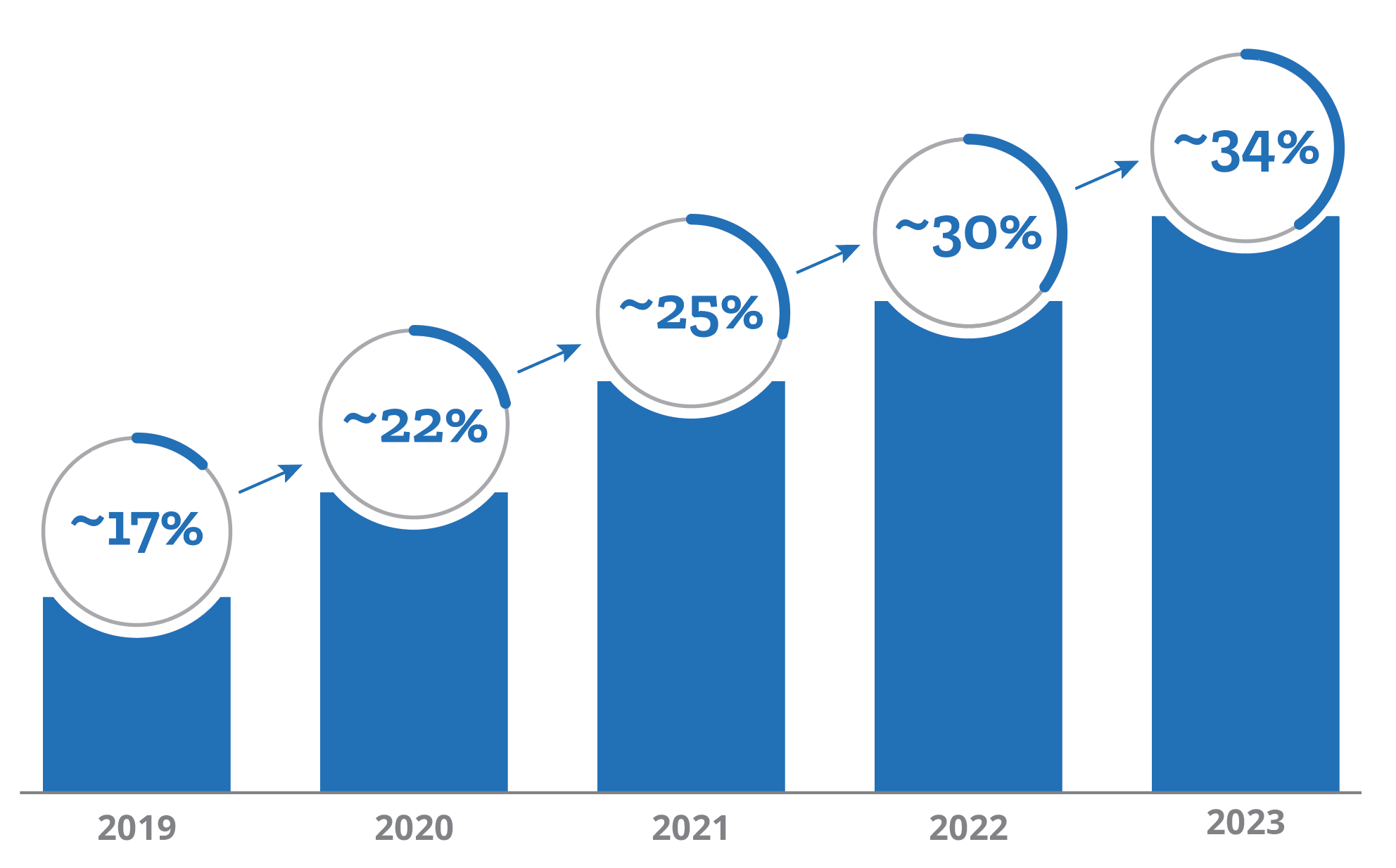
49
By developing high-value solutions using our proprietary intellectual property, we are able to create exclusive products, processes and services that can be used across different clients. For example, our “high- value” drug containment solutions, such as ALBA® and NEXA®, are particularly well-suited to address the needs of customers in the biologics end market, as they:
The strong relationships we have developed with our customers and our ability to work alongside them across each stage of the drug development process, from pre-clinical to clinical stage and commercialization, allow us to understand their specific needs at an early stage of the drug development and production process and provide appropriate solutions for such needs. Our strong relationships, our ability to provide a full set of solutions across the drug development process, and our expertise in developing and assembling machinery and equipment for the production of drug containment and delivery systems make us a partner of choice for our customers.
Drug Containment Solutions (DCS)
DCS are mission-critical components in the production of pharmaceutical and biotechnology products. Our drug containment systems are complex and rely on multiple sophisticated industrial processes to form, treat, inspect and package these products. We provide high-value DCS in pharmaceutical grade glass and in polymer materials such as cyclic olefin polymer (COP) and cyclic olefin copolymer (COC).We believe that the breadth and variety of our DCS offering represents one of our key competitive advantages. Our portfolio of DCS products includes:
50
High Value Solutions: Our DCS portfolio comprises several innovation-driven “high-value” solutions, illustrated below:
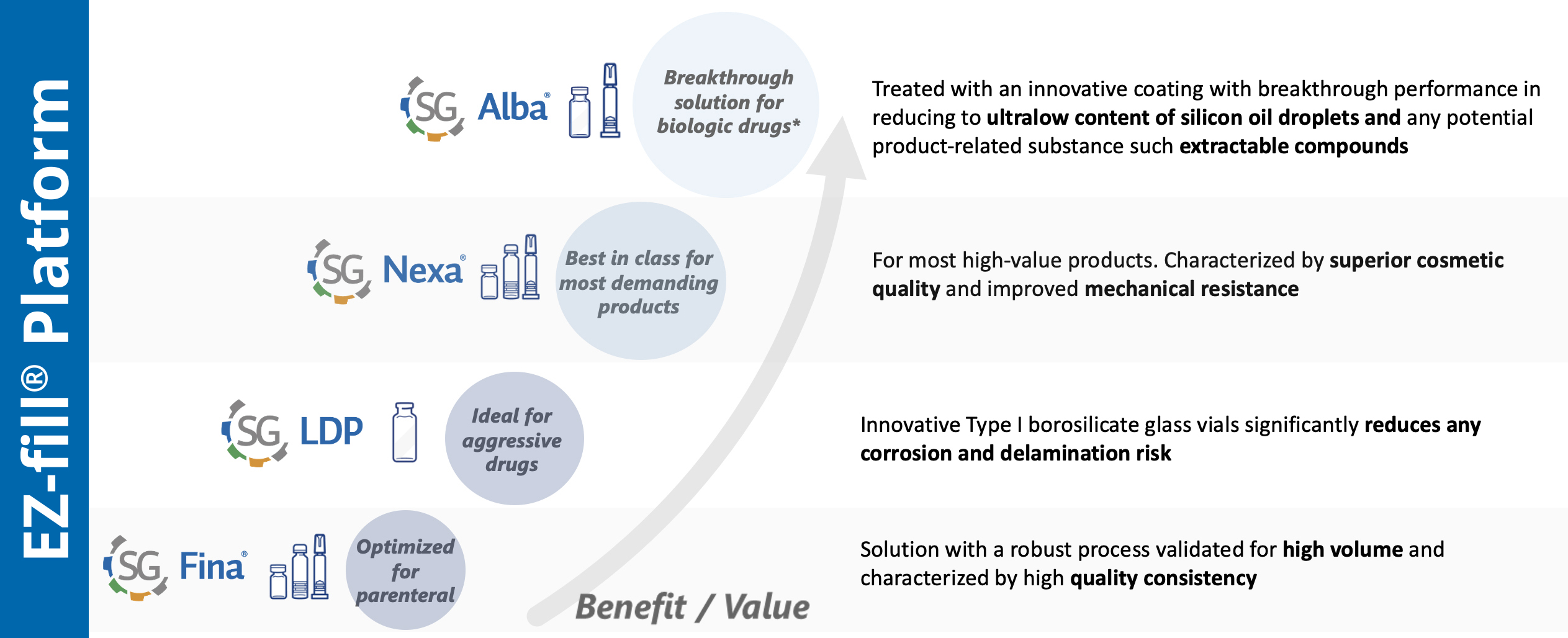
Our most innovative DCS solutions, some of which include integrated safety systems, are:
51
In-Vitro Diagnostics (IVD) solutions
Within the life sciences industry, we specialize in the development and manufacturing of customized diagnostic laboratory consumables (Point-of-Lab), as well as diagnostic consumables for use outside of laboratories (Point-of-Care) and IVD systems. These products are used in laboratories, hospitals, primary care facilities and in-home care settings on a worldwide scale.
The life sciences sector is complex as it requires constant cooperation with each customer for the development of the specific products they need. Whereas in the production of DCS we independently develop the shape and size of each container in accordance with customers’ instructions, the production of IVD solutions requires development of specific molds based on each customer’s requirements and specifications, which are then used for stamping of the final product. The development of these molds is a lengthy process that requires close cooperation with the customer and results in the customer retaining ownership of the mold(s). We intend to develop this business into an integrated platform covering all parts of the process, from product development to delivery of the final product, packaged and sterilized as needed.
Drug Delivery Systems (DDS)
Our DDS offering includes the following four product categories:
52
We also provide contract development and manufacturing services for customer-owned drug delivery devices, including design, manufacturing, industrialization, component manufacturing and high-precision injector molding and assembly.
Analytical Services and Regulatory Support

We have two analytical testing facilities in Piombino Dese, Italy and Boston, Massachusetts focused on investigating the physical and chemical properties of primary packaging materials and components. By studying the interaction between drug containment solutions and drug products we provide valuable data to customers toward the definition of the optimal drug containment or delivery solution. This allows us to engage with our clients earlier in the development phase of their drugs and position ourselves well to become a supplier for their containment solution and potentially their drug delivery systems and related process equipment.
The containment and delivery solution that we provide is an integral part of the drug product itself and it is included as part of the regulatory filings required before commercialization. We also assist our customers in this phase of their drug product development by providing the analytical and scientific support required to obtain the relevant regulatory authorizations.
Our key analytical services, supported by our regulatory know-how, include:
53
Engineering Segment

Our engineering segment produces machinery for both in-house use and sale to customers. In our Piombino Dese (Italy), Bologna (Italy) and Silkeborg (Denmark) plants, we produce equipment and machinery for all phases of the glass production process, as well as for the assembly of plastic products and for the inspection of primary containers. We drive continuous technological advancements so that our equipment can consistently meet our client’s exact specification requirements. Our engineering services span all phases of the machinery production process from development and design, including the development of software and artificial intelligence models, to construction, assembly and testing. With approximately 170 specialists and technicians located worldwide, we provide after sales support to our customers with interactive tools and technical expertise, to ensure performance of their production sites. We are currently engaging in initiatives to consolidate our engineering operations in order to streamline our operations and improve operational efficiencies.
Our engineering products include:
54
We also provide professional project management services, supporting our customers in designing their plant layout for the production of bulk and ready-to-use pharmaceutical and biotechnology primary packaging.
As a result of the experience gained designing our greenfield plants in Italy, China, Mexico and Brazil, our offering includes support and consultancy around: (i) plant design, (forming lines, clean room areas and laboratory layout); (ii) plant construction (production flow, piping and instrumentation diagrams); and (iii) plant engineering (preliminary plant studies).
Customers
We serve a large and diversified customer base of more than 700 companies worldwide, including many of the world’s largest pharmaceutical and biotechnology companies, life sciences companies, drug product and fill and finish contract manufacturers.
Our customer base includes all of the top 25 pharmaceutical companies, and eight of the top ten in-vitro diagnostic companies, as measured by 2022 revenue, according to data collected by Pharmacircle and public companies’ information. We also serve seven of the top ten biotechnology companies by market capitalization in the Nasdaq Biotechnology Index and over 100 biotechnology customers in total.
Drug containment and delivery solutions are an essential element in our customers’ manufacturing processes but generally represent a small fraction of the total cost of producing drugs. We therefore believe our customers choose our products, processes and services based on quality, reliability, innovation, speed to market and consistency rather than on costs.
We seek to maintain high levels of engagement with our customer base in order to deepen our relationships over time. Our deep, tenured relationships with our customers are supported by multi-year contracts which often contain cost pass-through provisions and have resulted in large recurring revenue streams. We engage with our customers through a variety of touchpoints, including direct visits, third-party and proprietary educational events, webinars, digital and social media communication channels designed to gauge consumer satisfaction with our products, technologies and services.
In 2023, our ten largest customers accounted for 45.5% of our consolidated revenue, and one customer accounted for 11.4% of consolidated revenue.
Customer Categories
We believe that quality, breadth of services and innovation are the main factors enabling us to deliver significant value to our customers making us a partner of choice for them. Our main customer categories include:
Pharmaceutical and Biotechnology Companies
Our pharmaceutical and biotechnology customers include large, international companies, as well as smaller regionally focused companies and manufacturers. We provide a diverse range of products, processes and services to these customers, both within our Biopharmaceutical and Diagnostic Solutions segment and our Engineering segment. Over time, we have invested in developing innovative products, services and solutions to serve pharmaceutical and biotechnology customers, which has enabled us to form long lasting relationships underpinned by the reliability and quality of our offering, processes and services.
The validation process for suppliers of pharmaceutical and biotechnology solutions, both with regard to drug containment and delivery systems and engineering, requires rigorous evaluation of multiple quality and compliance criteria and can sometimes last several years.
For this reason, we believe that we are well-positioned to enjoy durable long-lasting relationships with our customers, as we are deeply embedded in their production processes.
55
Life Sciences Companies
Our main life sciences customers are in-vitro diagnostic companies. We provide life sciences companies with contract development and manufacturing services for the plastic consumables used in their diagnostic tests and containment solutions for their reagents as well as machinery for the production, assembly and visual inspection of such products.
Drug Product / Fill & Finish Contract Manufacturers
We provide our solutions to drug product / fill & finish contract manufacturers. We provide these customers with glass and plastic containers as well as engineering solutions for the assembly, visual inspection, secondary packaging and serialization of their products.
Customer Service
We have a customer service team that works in parallel with the sales, supply chain, operations, technical and quality teams at our plants to collect feedback at every stage of our production process. Our centralized customer service is headquartered in Italy to ensure that our global team of customer service professionals applies consistent processes and procedures to guarantee high-quality and superior service levels.
Customer Contractual Arrangements
We have different contractual arrangements for different business segments. In our Biopharmaceutical and Diagnostic Solutions segment, our relationships with our customers are governed typically by master supply agreements the terms of which apply to each purchase order or product schedule through which customers place their request for the supply of our products. These are normally multi-year contracts generating consistent and recurring revenue streams, often times over the life cycle of a drug. We negotiate different master supply agreements with each customer and, although similar, there are no standardized terms across all agreements. Many of our agreements contain provisions which allow us to pass through cost increases from raw materials, logistics or energy.
Sales in our Engineering segment relate to individual machinery and contracts and are therefore negotiated on an individual basis. Pursuant to these equipment sale agreements, the intellectual property rights developed during the production of the relevant equipment and services remain our exclusive property save for cases of co-development with our customers.
Backlog
Our backlog represents, as of a point in time, estimated future revenue for work not yet completed under (i) specific purchase orders, with regards to our Biopharmaceutical and Diagnostic Solutions Segment; and (ii) certain one-off agreements, with regards to our Engineering Segment. As of December 31, 2023, our total backlog was approximately €944.9 million compared to €957.0 million as of December 31, 2022.
In our Biopharmaceutical and Diagnostic Solutions segment we generally operate under long-term supply and/or framework agreements. Our backlog represents, as of a point in time, estimated future revenues from work not yet completed under specific purchase orders.
In our Engineering segment we generally have one-off agreements and our backlog represents, as of a point in time, estimated future revenues from work not yet completed under those agreements.
To the extent projects are delayed, accelerated, or changed the timing of our revenue could be affected. If a customer cancels or modifies an order, we may be reimbursed for the costs and often for the missing profit we have incurred. For orders that are placed inside a contractual firm period, we generally have a contractual right to payment in the event of cancellation. Fluctuations in our reported backlog levels also result from the timing and order pattern of our customers who often seek to carefully manage supply chain risk and their level of inventory on hand. Because of customer ordering patterns, our backlog reported for certain periods may fluctuate and may not be indicative of future results.
56
Competition
We compete across a broad spectrum of products, processes and services for integrated containment and delivery solutions as well as engineering solutions. The breadth of our integrated capabilities differentiates us from our competitors as we believe we are the only player to be active in both the drug containment and delivery systems and engineering segments. We maintain constructive relationships with our competitors and in some cases, we acquire production inputs for our primary packaging from them. Similarly, we sell our equipment and machinery and license certain intellectual property rights to competitors for use in their production processes.
Given the breadth of our offering, we have different competitors for different products, and in particular we consider the main competitors in each segment to be:
Marketing
We market and sell our products both directly and, to a lesser extent, through a limited number of third- party partners globally.
Our salesforce is organized both vertically, by geography (Americas, EMEA and APAC) and key accounts, and horizontally, by business activity (technical pre-sales, product managers, aftersales and business development). Our sales team, of approximately 160 employees, works closely together in each area and region to ensure a coordinated approach.
We typically establish and maintain long-standing, direct relationships with all our customers and our salesforce proactively engages with our current and prospective customers to continuously share information and evaluate their needs, so that we may tailor our solutions in real time. Additionally, we host a series of events such as our Innovation Day and Pharmapack Symposium to provide a forum for collaboration and exchange of ideas with our customers. We work with and learn from our customers, and we develop products around their specific needs to address requirements and market demands.
Manufacturing, Facilities and Supply Chain Overview
Our Approach to Manufacturing
Our manufacturing approach is based on the relentless pursuit of maximum efficiency and highest quality. We implement top of the line hardware and software solutions to deliver consistent quality standards, in an attempt to minimize human impact in the production chain. Our manufacturing methods and processes, which are standardized in all of our production facilities, allow us to provide consistent products, processes and services, both in terms of quality and time to market, worldwide. Our pro-active, forensic approach to manufacturing together with our careful oversight allow us to mitigate the risk of quality issues to customers, thereby reducing additional costs for them and us. At all stages of our manufacturing process we strive to preserve the integrity of medicines and protect the safety of patients.
Our ultimate goal is to achieve “zero quality issues” by delivering products and services that never fall short of client specifications, and by operating a quality control system capable of preventing a sub-standard product from ever being delivered to our customers.
57
RAFT (Right At First Time) execution, respect for deadlines and flawless processes enable us to achieve very high customer satisfaction, while fostering loyalty and enhancing our reputation. Moreover, the combination of our scientific skills and engineering capabilities, which makes us a unique player in the market, enables us to minimize waste and maximize efficiency.
Digitalization along the manufacturing chain paves the way to make processes faster and more efficient and reduce defective products. Continuing technological innovation allows us to improve process robustness and increase output. By integrating different production steps like injection molding and assembly, we have successfully eliminated intermediate stocks, thereby realizing significant cost savings.
Our glass manufacturing process consists of four main steps:
Our EZ-Fill® process for syringes, vials and cartridges consists of five main steps:
Following the washing phase, the process is in an ISO 5 environment through final packaging. The in-process and quality controls for batch release are performed through specific control plans.
Our In-vitro Diagnostics, Medical Solutions and Drug Delivery Systems manufacturing process consists of six main steps:
58
Our engineering manufacturing process consists of nine main steps:
59
Facilities Overview
Below is a full list of our production facilities divided by business segment.
Biopharmaceutical and Diagnostic Solutions |
|
Location |
Product(s) |
Piombino Dese, Italy |
Primary Packaging (Vials, Cartridges, Syringes, EZ-Fill®) |
Latina, Italy |
Primary Packaging (Cartridges, Syringes EZ-Fill®) |
Bratislava, Slovakia |
Primary Packaging (Vials, Ampoules) |
Monterrey, Mexico |
Primary Packaging (Vials, Cartridges) |
Zhangjiagang, China |
Primary Packaging (Vials, Cartridges) |
Sete Lagoas, Brazil |
Primary Packaging (Vials, Cartridges, Ampoules) |
Bad Oeynhausen, Germany |
Medical Devices and components for Biopharma and Diagnostics |
Ontario, California, U.S. |
Medical Devices and components for Biopharma and Diagnostics |
Fishers, U.S. |
Primary Packaging (Syringes EZ-Fill®) |
Engineering |
|
Location |
Product(s) |
Piombino Dese and Bologna, Italy |
- Glass Converting Equipment - Assembly and Packaging equipment lines - Visual Inspection Equipment |
Aarhus, Denmark |
- Visual inspection Equipment |
Silkeborg, Denmark |
- Assembly equipment and lines - Packing equipment and lines |
Martellago, Italy |
- Mechanical components for industrial machines |
New facilities
We engaged in the construction of new plants primarily for the production of our EZ-Fill® product suite in Indiana (U.S.) and Latina (Italy). We have future plans to expand in Zhangjiagang (China) but have slowed the project to focus efforts on the execution of our plants in the U.S. and Italy.
On October 4, 2021 we announced the construction of a new EZ-Fill® hub in Fishers, Indiana. The plant, will enable us to be in closer proximity to our North America pharmaceutical customers and to provide an additional supply source. The facility will house production lines to produce EZ-Fill® syringes and vials. In line with customer demand and as a result of our increased production capacity, we expect to better support customers’ needs for biologics and vaccine treatments. The Indiana hub will also house our after-sales support services which will be dedicated to serving our North America engineering customers. Construction on the new plant is nearing completion, customer validation activities have commenced and we currently expect to launch commercial production in 2024.
In 2022, we elected to accelerate our investment in the United States in response to increased demand from customers. Under the refined investment plan, the manufacturing facility is expected to be up to 565,000 square-feet and with more than 500 new full-time positions. As part of this capital project, in February 2022, we entered into an agreement with the U.S. government’s Biomedical Advanced Research and Development Authority (BARDA), which is part of the U.S. Department of Health and Human Services, through its partnership with the U.S. Department of Defense. Under the agreement, BARDA will make a multi-year investment for up to approximately $95 million (or approximately €85 million) for manufacturing capacity for standard and EZ-Fill® vials in support of U.S. national defense readiness and preparedness programs for current and future public health emergencies.
On May 27, 2022, we acquired a brownfield in Latina (Italy) for a total consideration of approximately €16 million. The facility commenced commercial production beginning in the fourth quarter of 2023. When fully ramped, the facility is expected to produce EZ-fill® syringes and vials in response to market needs for ready-to-use drug containment products.
60
In December 2021, we signed the contract for the acquisition of an existing facility in Zhangjiagang, China for a new plant. In 2023, we decided to delay the timing of our investment in China to prioritize our expansion in the U.S. and Europe in order to satisfy expected future demand in those high-growth markets.
Seasonality
Our business and results of operations are not materially affected by seasonal fluctuations in the consumption of our products.
Supply Chain
We maintain positive relationships with suppliers across our business. The types of consumables we require differ by product as follows:
For some of the materials we use in our production cycles, we have a limited number of (or a single-source) suppliers worldwide, and selecting new suppliers would be a lengthy and time-consuming process. We have long-term agreements with several of our customers that allow us to recover increased costs related to certain expenses.
Supply Agreements with Schott and NEG
Schott and NEG accounted for a significant percentage of our glass tube supply in 2023.
In 2017 we entered into a Master Supply Agreement with Schott for the supply of glass tubes which was replaced by a new Master Supply Agreement dated November 21, 2019 and effective as of January 1, 2020. Under the agreement, we are required to purchase minimum quantities. Under the Schott Master Supply Agreement, we must notify Schott in October every year of the desired quantity to be purchased the following year. If the required quantity exceeds the minimum quantity imposed on Stevanato under the agreement, Schott approval is required. The agreement also provides for certain price adjustment mechanics based on manufacturing costs. The agreement expires on December 31, 2024 and contains customary termination provisions, allowing for termination by a party upon a material violation of the agreement or change of control by the other party. We are currently discussing plans to renew the agreement.
On October 24, 2019, we entered into a Supply and Purchase Agreement with NEG for the supply of glass tubes which became effective on January 1, 2020. The agreement has a term of three years at the end of which the parties may negotiate the terms of the renewal. On December 16, 2022, agreement was renewed for an additional three-year period (2023 through 2025). Under the agreement, we are required to purchase minimum quantities and NEG is required to supply minimum quantities. The agreement contains customary termination provisions, allowing for termination upon the other party’s default not cured within 30 days and bankruptcy, dissolution, suspension or other similar event.
61
Quality control
Providing high-quality products with specificity, sensitivity and consistency, coupled with extensive product validation data are fundamental drivers of customer loyalty. Customers in our target markets are particularly sensitive to products failing to meet specifications shown on data sheets. Our success depends on our customers’ confidence in our ability to provide reliable, consistently high-quality products, which includes our ability to provide validated data to support our customers’ use of our products. In this respect, we believe that our ability to provide consistent quality standards in each of our production facilities, due to our standardized production processes, allow us to win our customers’ trust and reliance.
Biopharmaceutical and Diagnostic Solutions
All of our facilities use efficient quality control and quality assurance procedures comparable to those used in the pharmaceutical and biotechnology industries at each stage of the manufacturing process. We are certified according to applicable ISO standards. We are subject to rigorous audits by certification bodies and our customers, who perform more than 150 audits a year on our manufacturing facilities. Our control procedures in our glass manufacturing facilities focus on physical and chemical characteristics, dimensional aspects and product appearance and are carried out on each of: (i) the glass tubes and raw materials; (ii) the various process phases of production; and (iii) the finished products. Along the production line and before packaging each product undergoes an automatic inspection for cosmetic defects. Defective pieces are discarded. Inspection is carried out with highly sophisticated electronic devices using specific defect detection algorithms. At the end of the production line, products are checked using statistical control procedures to test their quality for specific cosmetic, chemical, physical and dimensional parameters. Each of our plastic manufacturing facilities also follows similar quality control procedures, albeit specific to plastic production. The control procedures include dimensional and functional tests, focused on the mechanic and cosmetic features of the products. In particular, checks are carried out on each of: (i) the raw materials; (ii) dyestuff, additives and components; (iii) the various process phases; (iv) semi-finished products; and (v) finished products.
Engineering
Our quality control systems and related activities are designed to ensure that our manufacturing processes, as well as those of our pharmaceutical customers and the contract manufacturing companies we rely on comply with Good Automated Manufacturing Practice (GAMP) standards based on the GAMP guidelines issued by the International Society for Pharmaceutical Engineering (ISPE). Each individual piece of machinery/ equipment is developed and manufactured as a project, and ad hoc project management tools are utilized to manage every stage and minimize risk.
Our quality activities follow a Stage Gate Model which includes the following ten stages: (i) quotation: based on customer’s needs (URS—user requirements specifications); (ii) start-up: intended product use is defined by the subject-matter expert (SME), product risks are assessed through a failure mode and effect analysis (FMEA) and documented in accordance with relevant function specifications; (iii) design: design of the equipment based on the FMEA is reported in a specific format, including a traceability matrix that ties back to the user requirements specifications; (iv) production base: prepare necessary documentation and release specific bill of materials, whose accuracy is assessed based on ad hoc checklists; (v) assembly: all components are sourced from approved suppliers and subsequently checked ahead of distribution to the assembly line. The relevant equipment is then assembled, the relevant software installed and all connections are checked and tested based on ad hoc checklists; (vi) running-in: the equipment is completed and test plans are prepared; (vii) FAT: systematic verification with predefined test based on FMEA risk assessment for the specific design. Testing covers all relevant customer’s requirements; (viii) commissioning: the equipment is transported to customer’s facility and installed based on pre-agreed requirements and specifications set out in ad hoc checklists; (ix) SAT: systematic on-site verification according to pre-defined protocol and reporting to customer; and (x) closing: project evaluation and monitoring to ensure continuing improvements.
Logistics
Our products are delivered to our customers using various third-party freight, haulage transportation and warehousing providers. Each manufacturing facility has its own logistics team that is responsible for managing product storage and delivery accounts. The pandemic has put increased pressure on logistics networks. As a result, we have
62
experienced slower delivery times and increased logistical costs related to incoming materials and delivery of our products.
Shareholder Agreement with SVM Holding ApS
In October 2021 we acquired the remaining 35% of the subsidiary SVM Automatik A/S (now Stevanato Group Denmark). Such acquisition resulted in the termination of the shareholder agreement with the minority shareholder SVM Holding ApS.
JV Agreement with SE Holdings Co. Ltd
In July 2023 we acquired the remaining 49% of the subsidiary Ompi of Japan Co. Ltd. Such acquisition resulted in the termination of the JV agreement with the minority shareholder SE Holdings Co. Ltd.
Intellectual Property and Information Technology
Intellectual Property
Our products, both in the Biopharmaceutical and Diagnostic Solutions and in the Engineering segments, are highly sophisticated and based on the development of specific know-how, processes and procedures. We actively protect our intellectual property rights and know-how through patents, trademarks and trade secrets. We actively map and protect our trade secrets and thus the technology behind our core products and processes is protected by 85 patent families, the most important ones being those devoted to the protection of the EZ-fill® solutions, the pre-sterilized drug containment solutions for aseptic manufacturing.
Our most distinctive brands are also protected via registered trademarks, the most important being: (i) SG-toothed wheel logo, (ii) EZ-fill®, (iii) EZ-fill SMART™ (the next generation EZ-fill® product suite) (iv) NEXA® (relating to superior drug containment solutions for mechanical resistance and cosmetic quality), (v) ALBA® (relating to advanced drug containment solutions for optimized drug-containment interaction), (vi) Vertiva™ (relating to wearable injectors), (vii) Alina® (relating to pen injector devices).
Information Technology
We keep strengthening our IT framework focusing on improving IT security and best practices. We are reviewing and updating our IT policies to the latest requirements to protect the Group’s information. In 2017, we began our digital transformation process to facilitate the Group’s growth in a globally competitive market. Our goal is to further improve, expand, and develop our digital ecosystem through 2024. To date, we have:
Through 2024, we intend to continue to:
63
Our IT infrastructure is hosted by Telecom Italia in a data center in Padua and is replicated in Microsoft Azure Cloud. The data center is connected to Amazon Web Services and Microsoft Azure cloud networks, based in Frankfurt and Amsterdam respectively. Our Group companies are connected to the data centers through multiprotocol label switching networks.
We have also continued to implement a cybersecurity improvement program to strengthen our existing IT security, foster greater cyber resilience of our IT systems, and improve our business continuity and disaster recovery procedures. As part of our cybersecurity improvement program, we have:
We will continue to further strengthen our cybersecurity program in 2024. We are not aware of any material cybersecurity incident over the past years, nonetheless the company is covered by a cybersecurity insurance plan. For a description of the cybersecurity risks we may be facing see “Risk Factors – Risks Relating to our Business and Industry – Cyber security risks and the failure to maintain the confidentiality, integrity and availability of our computer hardware, software and internet applications and related tools and functions, could result in damage to our reputation, data integrity and/or subject us to costs, fines or lawsuits under data privacy or other laws or contractual requirements” and “ITEM 16K. Cybersecurity”.
Research and Development
Research and development investment is a fundamental component of our growth and continued success. Our research and development team comprises more than 150 highly skilled and specialized employees operating in Italy, Germany and the U.S.
The goal of our research and development effort is twofold: (i) to facilitate the transition from hospital to home care; and (ii) enabling biologics to reach the patient safely by meeting the most critical quality and performance requirements.
We pursue these goals by investing in both the Biopharmaceutical and Diagnostic Solutions segment (with the exception of in-vitro diagnostic products in relation to which we do not conduct research) and the Engineering segment. In 2023, for example, our research and development expenses amounted to €35.7 million, the majority of which was in connection with our Drug Delivery Systems business and our high-value solutions pipeline.
Our Biopharmaceutical and Diagnostic Solutions research mainly focuses on DDS patient-centricity, sustainability and digitalization and improvement of DCS production processes and coating systems. In this segment we conduct our development activities both autonomously and, to the extent our customers require specialized or customized products, in close cooperation with them. We are most frequently asked to produce specialized or customized products by our pharmaceutical and biotechnology clients (in both glass and plastic).
In particular, in the area of containment solutions, the development of new products will be targeted at maintaining the stability, potency and purity of our customers’ products prior to administration. New therapies for diabetes, cancer treatment and auto immune diseases are based on large, complex bio molecules that are extremely sensitive to their storage environment. In the area of drug delivery systems, we will be targeting the development of easy-to-use, accurate, reliable self-injection systems for complex pharmaceutical and biotechnology products. We have developed a portfolio of devices for this market that can be used off-the-shelf or customized to the specific needs of the customer.
We will continue developing new drug delivery systems based on three pillars: patient centricity, sustainability and digitalization, core capabilities to meet our customer’s need for connected health devices. We apply a rigorous “stage & gate” development process, which de-risks our development projects and reduces total development costs. Development timelines for new drug delivery devices typically fall into the range of four to five years to reach the start of initial production. We cooperate with third parties on joint development projects. Pursuant to the relevant joint
64
development agreements with these third parties, we either own, or are entitled to co-ownership of or license rights to, the intellectual property rights developed in connection with these programs.
Our main focus in Engineering is on maximizing our machine performance while reducing total cost of ownership. Further, we are broadening our portfolio of products, processes and services with the aim of creating a stable platform able to minimize planned and avoid unplanned downtime, and developing and integrating artificial intelligence into our machinery.
In certain research areas, including chemical-physical and morphological characterization of glass surfaces and interactions with drugs, we cooperate with Universities such as Ca’ Foscari University (Venice, Italy), and Federico II University (Naples, Italy). We also have a research collaboration with the University of Colorado to focus primarily on interactions and compatibilities of primary packaging with biologics. Pursuant to the relevant cooperation agreements with these Universities, we either own, or are entitled to co-ownership of, the intellectual property rights developed in connection with these programs.
Employees
As of December 31, 2023, we employed 5,635 employees in multiple jurisdictions (approximately 56% based in Italy, 9% based in Germany, 8% based in Mexico, 6% in each of Slovakia, the U.S. and Denmark each, 5% based in Brazil and 4% based in China). The following table provides a breakdown of employees across the various main departments.
Department |
|
Total |
|
|
Direct Labor |
|
|
3,467 |
|
Industrial / Manufacturing Overhead |
|
|
1,459 |
|
G&A—Corporate Functions |
|
|
205 |
|
Sales & Marketing |
|
|
169 |
|
Research & Development |
|
|
151 |
|
G&A—Accounting Finance Control |
|
|
98 |
|
Human Resources |
|
|
77 |
|
CEO Office |
|
|
9 |
|
Our success largely depends on the skills and experience of our management, including our Executive Chairman, Chief Executive Officer and our senior leadership.
Our excellence in manufacturing processes derives in part from our employees mastering specific techniques and know-how. Certain roles, such as engineers, designers, quality controllers, can also require lengthy training due to the highly technical and diversified nature of the processes used in our production.
A substantial majority of our employees are covered by collective bargaining or similar agreements, which require periodic renegotiation. We believe we have strong relationships with our employees. We have not experienced any material work stoppages or strikes at any of our manufacturing facilities in recent years. We take a constructive approach to relationships with trade unions and works councils.
Insurance
We maintain product liability, property, cybersecurity and other insurance coverage to the extent we believe necessary to operate our business. We believe that our liability insurance is sufficient to meet our needs in light of expected possible future litigation and claims. We monitor regularly our risk profile and adjust coverage accordingly.
65
LEGAL PROCEEDINGS
We are involved, from time to time, in various litigation and administrative and other legal proceedings, including potential regulatory actions, incidental or related to our business, including commercial contract and tortious liability claims, among others (collectively, “Legal Proceedings”). While we cannot predict any final outcomes relating thereto, management believes that the outcome of current Legal Proceedings will not have a material effect upon our business, financial condition, results of operations, cash flows.
However, management’s assessment of our Legal Proceedings is ongoing, and could change in light of the discovery of additional facts with respect to Legal Proceedings pending against us, not presently known to us, or determinations by judges, arbitrators, juries or other finders of fact or deciders of law which are not in accord with management’s evaluation of the probable liability or outcome of such Legal Proceedings. From time to time, we are in discussions with regulators, including discussions initiated by us, about actual or potential violations of law in order to remediate or mitigate associated legal or compliance risks. As the outcomes of such proceedings are unpredictable, the results of any such proceedings may materially affect our reputation, our business, financial condition, results of operations, cash flows.
Under Italian Law, directors and officers (in their capacity as employers) have a duty of care towards their employees and are therefore responsible for their health and safety. Breaches of this duty of care can result from any non-compliance or accident occurred within the facility, regardless of an actual act or omission of such directors, as they are strictly liable in light of their role (posizione di garanzia).
Some of our directors have been subject to criminal proceedings in connection with their roles as employers, either for the Stevanato Group or for one of its subsidiaries, and in particular:
Regulations
The following paragraphs provide a brief description of the primary Italian, European and international laws and regulations that govern our activities. References and discussions to laws, regulations, directives and treatises and other regulatory acts are entirely qualified by the full texts of laws, regulations, directives and treatises, other administrative and regulatory acts themselves.
Health and safety
At all of our locations, we are subject to national laws, regulations and practices concerning employee health and safety. While each site is responsible for monitoring compliance with local regulations, we have a health and safety
66
network that operates across all of our manufacturing facilities in order to share and promote best practices. Each of our manufacturing facilities is regularly audited and any corrective action required to maintain our global standards is implemented. To date, we have not been subject to any significant fines, penalties or other liabilities under laws and regulations relating to employee health and safety. However, there can be no assurance that we will not be subject to fines, penalties or other liabilities in the future or that changes in such laws and regulations, or interpretations thereof, will not have an adverse impact on our operations.
Product safety regulation
The use, manufacture and importing of chemicals is highly regulated in the European Union. On June 1, 2007, Regulation 1907/2006 concerning registration, evaluation, authorization and restriction of chemicals (“REACH”) entered into force. Our products and the raw materials we use in our production processes are subject to various regulations related to product and chemical safety, including the REACH regulation in the European Economic Area. REACH requires that certain substances imported or manufactured within the European Economic Area be registered with the European Chemicals Agency and evaluated for safety. The registration process requires producers to generate and submit data on the environmental and health impacts of substances and, in some cases, obtain authorization for their use within the European Union. Among other things, REACH can result in the imposition of use or marketing restrictions, and may require the phase-out or substitution of certain more dangerous chemicals with suitable alternatives. The European Union is continually adopting additional requirements related to product or substance safety. Although REACH compliance is primarily the responsibility of our suppliers or the producers of chemical raw materials, we are also affected by REACH as a “downstream” user of REACH-regulated substances.
It is possible that the registration process or use restrictions imposed by REACH could increase our costs, affect our raw material supplies or require us to substitute certain materials with alternatives. We utilize a database system that allows us to track and monitor our suppliers and the REACH-compliance status of raw materials used at each of our facilities. We biannually review official databases to ensure that our suppliers have made the required registrations and are in material compliance with REACH, and check that they have efforts underway to prepare for and comply with any additional requirements or upcoming deadlines. We believe that we have the capability to adjust our products and supplies as needed in accordance with any future requirements of REACH.
Environmental
Our operations are subject to a number of European, national and local environmental laws and regulations relating to the protection of the environment and natural resources. These include laws and regulations relating, inter alia, to air and noise emissions and the impact made on air quality through gas and particle emissions, and recycling and packaging waste reduction and prevention. Compliance with these laws and regulations is monitored by local and national authorities and competent agencies and non-compliance with these laws can result in administrative orders, substantial fines and criminal penalties, temporary or permanent plant closures and criminal convictions. Our current and past operations, including our historical waste disposal sites, could also expose us to liability to third parties for property damage, personal injury and clean-up obligations. We believe that our manufacturing facilities currently comply, in all material respects, with the applicable material environmental regulations at each of our locations, and as of the date hereof, we are not aware of any environmental issues requiring investigation or remediation on our behalf. However, there can be no assurance that changes in such laws and regulations, or interpretations thereof, will not require us to incur significant costs, which could have an adverse impact on our operations.
67
A full list of our significant subsidiaries is shown in the following diagram and depicts our simplified organizational and ownership structure.
The following diagram illustrates our corporate structure as of the date of this annual report. We conduct business through several direct and indirect subsidiaries operating in Europe, Asia and the Americas.
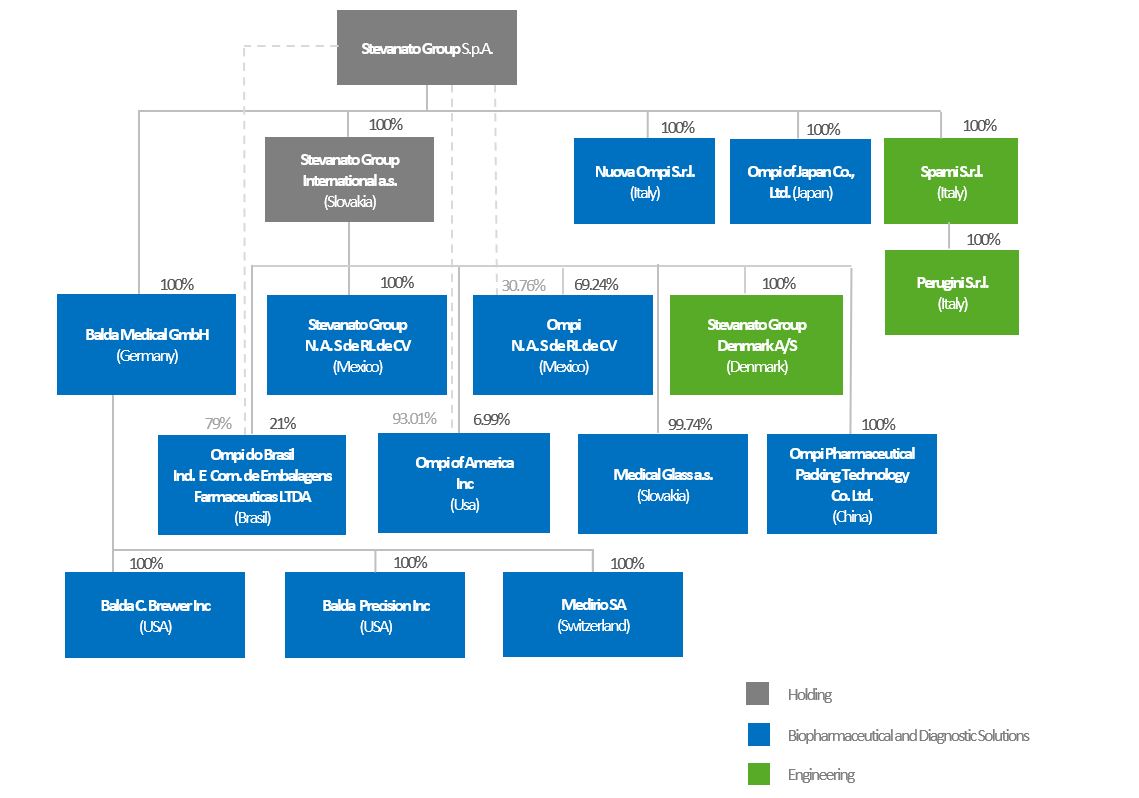
We are headquartered in Italy and our registered office is located in via Molinella 17, Piombino Dese (Padua, Italy). As of December 31, 2023, we had nine production plants for manufacturing and assembly of pharma and healthcare products (in Italy, Germany, Slovakia, Brazil, Mexico, China, United States), six plants for the production of machinery and equipment (in Italy and Denmark), two sites for analytical services (in Italy and United States) and two commercial offices (in Japan and the United States). See “—B. Business Overview—Our Business— Manufacturing, Facilities and Supply Chain Overview—Facilities Overview” for a table setting forth a full list of our production facilities divided by business segment as of December 31, 2023.
ITEM 4A. Unresolved Staff Comments
None.
68
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
You should read the following discussion of our financial condition and results of operations in conjunction with our consolidated financial statements and the notes included elsewhere in this annual report. The following discussion contains forward-looking statements that involve certain risks and uncertainties including, but not limited to, those described in the “Risk Factors” section of this annual report. Our actual results could differ materially from those discussed in these statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this annual report, particularly under the “Risk Factors” and “Special Note Regarding Forward-Looking Statements” sections. Certain information required by this ITEM 5, including a discussion of the year ended December 31, 2022 compared to the year ended December 31, 2021, has been reported previously in our Annual Report on Form 20F for the year ended December 31, 2022 filed with the U.S. Securities and Exchange Commission on March 2, 2023, under the section entitled “Operating and Financial Review and Prospects”.
A. OPERATING RESULTS
Overview
We are a leading global provider of drug containment, drug delivery and diagnostic solutions to the pharmaceutical, biotechnology and life sciences industries. We deliver an integrated, end-to-end portfolio of products, processes and services that address customer needs across the entire drug life cycle at each of the development, clinical and commercial stages. Our core capabilities in scientific research and development, our commitment to technical innovation and our engineering excellence are central to our ability to offer value added solutions to our clients.
We have secured a leadership position within the drug development and delivery value chain through our investment in research and development and the expansion of our global footprint and capabilities. Over our 70-year history, we have earned a leading reputation for high quality and reliability that has enabled us to become a partner of choice for more than 700 companies globally. Our customer base includes 25 of the top 25 pharmaceutical companies, and eight of the top ten in-vitro diagnostic companies, as measured by 2022 revenue, according to data collected by Pharmacircle and public companies’ information. We also serve seven of the top ten biotechnology companies by market capitalization in the Nasdaq Biotechnology Index and over 100 biotechnology customers in total.
Our priority is to provide flexible solutions that preserve the integrity of pharmaceutical products and enable our customers to deliver safe and effective treatments to patients while reducing time to market, total cost of ownership (i.e., logistics, drug product waste, storage and personnel costs) and supply chain risk. We achieve this by developing our products in close collaboration with our customers, leveraging our scientific research capabilities, technical expertise and engineering and manufacturing excellence to meet their quality requirements. For example, under our exclusive collaboration agreement with Owen Mumford, which we entered into in March of 2022, we are harnessing the full breadth of services to deliver our Aidaptus® auto injector device.
Our solutions are highly integrated with the development, production and commercialization processes of our customers. In addition to manufacturing drug containment and delivery solutions, we provide a full set of services across all stages of drug development, from pre-clinical to clinical and commercialization. We also engineer machinery and equipment for the production of drug containment and delivery systems that can be integrated into both our customers’ and our own manufacturing processes. Our involvement at each stage of a drug’s life cycle, together with the breadth of our offering, enables us to serve as a one-stop-shop for our customers, which we believe represents a significant competitive advantage.
We operate across the healthcare industry and serve some of its fastest growing segments, including biologics (including GLP-1s, monoclonal antibodies and mRNA applications), biosimilars, vaccines and molecular diagnostics. As a result of how closely integrated we are in the drug production and delivery supply chain, we are well-positioned to benefit from multi-year, secular trends within our target industries, such as increases in demand resulting from pharmaceutical innovation, acceleration and expansion of vaccination programs, growth in biologics/biosimilars, self-administration of medicines, aging demographics, increasing quality standards and regulation and a shift towards outsourcing non-core functions by our customers.
We estimate that our total addressable market, based on our current offering, exceeded $15 billion in terms of revenue generated by all market participants in 2023, and consists of biopharmaceutical injectables and in-vitro
69
diagnostic products. The addressable market estimation is based on revenue from all market players in 2023; if revenue from 2023 is not available, revenue for 2022 is used applying market growth rate from third parties such as IQVIA, Roots Analysis, Markets and Markets Research, and Alira Health. Within each of these markets, we operate in some of the fastest growing segments, including pre-fillable syringes, drug delivery systems, molecular diagnostics and assembly equipment.
We believe there are opportunities to further expand our addressable markets, including by targeting (i) complementary containment solutions, (ii) additional delivery systems, (iii) complementary engineering solutions, and (iv) aftersales support and services.
We operate our business in two segments:
For the years ended December 31, 2023 and 2022, we generated 81% and 81% of total sales from our Biopharmaceutical and Diagnostic Solutions segment, respectively and 19% and 19% from our Engineering segment, respectively.
We refer to our premium products in the Biopharmaceutical and Diagnostic Solutions segment as our “high-value” solutions. High-value solutions are products, processes and services for which we hold intellectual property rights or have strong proprietary know-how, and that are characterized by particular complexity and high performance. Our high-value solutions deliver significant benefits to customers including higher quality, reduced time-to-market and reduced total cost of ownership. These high-value solutions are typically pre-washed, sterilized and in a ready-to-use format. Presently, only about 5% of the vial market and less than 5% of the cartridge market has transitioned to a ready-to-use format, while 95% of the syringe market has transitioned to a ready-to-use pre-fillable syringes. However, we are currently experiencing a desire by customers to transition to ready-to-use formats to benefit from one or more of the above mentioned efficiencies to different extents. Among our key high-value solutions is our EZ-Fill® line of ready-to-fill injectable products, which can be customized to meet clients’ needs. For additional information on EZ-Fill® see “Business—Business Segments—Biopharmaceutical and Diagnostic Solutions— Drug Containment Solutions (DCS).”
We have nine production plants for manufacturing and assembling pharmaceutical and healthcare products across Europe (Italy, Germany and Slovakia) and the rest of the world (Brazil, China, Mexico and the U.S.), six plants for the production of machinery and equipment (Italy and Denmark), two sites for analytical services (Italy and the U.S.) and two commercial offices (Japan and the U.S.). Our manufacturing facilities in Mexico (serving the U.S. market), China and Brazil are greenfield operations established by us. Our manufacturing facilities in Slovakia, Denmark, Germany and the U.S. were acquired in strategic transactions over the past 15 years. Our global footprint, together with our proprietary, highly standardized manufacturing systems and processes, allow us to provide quality consistent products and services to our customers in more than 70 countries. The Group is expanding its global industrial footprint with capacity expansions in Fishers, Indiana, U.S., and in Latina, Italy, primarily to add capacity in its premium EZ-Fill® products to diversify its product supply and improve proximity to customers. In 2023, the Group elected to slow down its capacity expansion in China in order to focus efforts and resources on the ramp up of its manufacturing facilities in the U.S. and Italy where demand outpaced initial expectations. On November 8, 2023, we acquired Perugini S.r.l., an Italian company specialized in the manufacturing of consumables and mechanical components for industrial machines. The acquisition of Perugini will support the Group's efforts in the ongoing integration of critical technologies and processes into the Group’s production process.
70
Highlights
Consolidated Income Statement Data
|
|
(Amounts in € millions, except as indicated otherwise) |
|
|||||||||
|
|
For the year |
|
|
Change |
|
||||||
|
|
2023 |
|
|
2022 |
|
|
% |
|
|||
Revenue |
|
|
1,085.4 |
|
|
|
983.7 |
|
|
|
10.3 |
% |
Gross Profit |
|
|
339.9 |
|
|
|
319.8 |
|
|
|
6.3 |
% |
Operating Profit |
|
|
200.7 |
|
|
|
192.4 |
|
|
|
4.3 |
% |
Profit Before Tax |
|
|
189.6 |
|
|
|
187.6 |
|
|
|
1.0 |
% |
Net Profit attributable to: |
|
|
|
|
|
|
|
|
|
|||
Equity holders of the parent |
|
|
145.6 |
|
|
|
142.8 |
|
|
|
1.9 |
% |
Non-controlling interest |
|
|
0.1 |
|
|
|
0.2 |
|
|
|
(64.7 |
)% |
Basic earnings per common share (in €) |
|
|
0.55 |
|
|
|
0.54 |
|
|
|
1.9 |
% |
Diluted earnings per common share (in €) |
|
|
0.55 |
|
|
|
0.54 |
|
|
|
1.8 |
% |
Dividend approved per share (in €) (1) |
|
|
0.054 |
|
|
|
0.051 |
|
|
|
5.9 |
% |
Dividend approved per share (in $) |
|
|
0.058 |
|
|
|
0.055 |
|
|
|
5.5 |
% |
Following approval of the Stevanato Group S.p.A. Financial Statements at December 31, 2021 by the shareholders at the Annual General Meeting of the Shareholders on June 1, 2022, a dividend distribution of €0.051 per outstanding share was approved, corresponding to a total distribution of €13.5 million. This distribution was made in part from the net profits realized in the previous financial year and in part from "other reserves". In July 2022 the Company paid €13.5 million of the distribution to shareholders of record at June 14, 2022.
For further information on Earnings per share calculation, see “15. Earnings per Share” in the Consolidated Financial Statements.
Consolidated Statement of Financial Position Data
|
|
(Amounts in € millions) |
|
|||||||||
|
|
At December 31, |
|
|
At December 31, |
|
|
Change |
|
|||
|
|
2023 |
|
|
2022 |
|
|
€ |
|
|||
Assets |
|
|
|
|
|
|
|
|
|
|||
Total current assets |
|
|
861.9 |
|
|
|
845.8 |
|
|
|
16.1 |
|
Total non-current assets |
|
|
1,209.7 |
|
|
|
813.9 |
|
|
|
395.8 |
|
Total assets |
|
|
2,071.6 |
|
|
|
1,659.7 |
|
|
|
411.9 |
|
|
|
|
|
|
|
|
|
|
|
|||
Liabilities and equity |
|
|
|
|
|
|
|
|
|
|||
Total current liabilities |
|
|
574.4 |
|
|
|
462.5 |
|
|
|
111.9 |
|
Total non-current liabilities |
|
|
364.5 |
|
|
|
201.3 |
|
|
|
163.2 |
|
Total liabilities |
|
|
938.9 |
|
|
|
663.8 |
|
|
|
275.1 |
|
Equity |
|
|
1,132.6 |
|
|
|
995.9 |
|
|
|
136.7 |
|
Total liabilities and equity |
|
|
2,071.6 |
|
|
|
1,659.7 |
|
|
|
411.9 |
|
Major Factors Affecting Our Results of Operation
Our financial condition and results of operations have been, and will continue to be, affected by a number of important factors, including the following:
71
Increasing Market Share in Growing Markets
We are a key partner to leading companies in the pharmaceutical, biotechnology and life sciences industries, serving as one of the preeminent providers of drug containment, drug delivery, diagnostic and engineering solutions to these end markets. The demand for our solutions is driven, in part, by trends affecting the pharmaceutical, biotechnology and life sciences markets, such as the aging of the global population, the increasing incidence of chronic diseases (e.g., diabetes), continued innovation in biologic injectables, increasing access to advanced healthcare in developing and transitioning countries, broader demand for vaccine programs, increasing propensity of biotechnology companies to outsource non-core competencies and growth in self-injection systems where the primary container (i.e., glass containers) is integrated into the delivery device. We believe that as a result of our global footprint and deep-rooted cooperation with our customers, we have been and will continue to be able to anticipate such market trends and adapt our products and services offering to benefit from them. Our ability to continue to grow our revenue and increase our market share will depend, in part, on our continued ability to target fast-growing market segments and to introduce new products and technologies more efficiently than our competitors.
Shift in Sales Mix Towards “High Value” Solutions
We continue to increase our focus on our innovation platform to extend and improve our in-house proprietary product offering. Our “high value” products generate substantially higher revenues and profits than other containment and delivery solutions. We also believe that “high value” products will support continued market share expansion in research use markets while enabling us to extend our product offering, through industry partners, to clinical applications. We expect to continue to devote significant resources to increase the proportion of “high-value” solutions we offer by focusing on developing innovative new products, both as part of our existing portfolio and in complementary and adjacent markets.
COVID-19 Pandemic Update
We have been in the vaccine business for decades, serving as a partner for the distribution of a variety of vaccines worldwide. In 2020, the global COVID-19 pandemic caused both governments and private organizations to implement numerous measures to contain the spread of the virus. We experienced both positive and negative impacts from the COVID-19 pandemic.
COVID-19 provided a favorable tailwind due to our critical role in supplying glass vials and syringes to support the roll-out of the COVID-19 vaccine to approximately 90% of the marketed vaccine programs. The pandemic also had unfavorable impacts including decreases in non-COVID-19 related products, labor absenteeism and supply chain disruptions, among others. The Group continues to experience some volatility in certain pockets of the supply chain (such as electronic components) and lower demand in certain product categories including in-vitro diagnostics and some vial products due to high customer inventory levels.
In 2021, the Group's COVID-19 related sales peaked at approximately 14.7% of total revenue and have since steadily declined. In fiscal years 2022 and 2023 we estimated COVID-19 represented approximately 11.2% and 1.6% of total revenue respectively. Currently, the Group expects revenue from COVID-19-related products and services may likely continue to decrease in fiscal year 2024 compared to fiscal year 2023. Longer-term, there remains uncertainty around the magnitude of demand for COVID-19 related products, however we currently expect demand for products related to COVID-19 will decrease and they will become part of our normal vaccine business as COVID-19 moves into an endemic stage.
Throughout 2023 we experienced lower vial volumes and revenue as customers continue to work down inventories that they stockpiled during the pandemic. The higher inventories are not limited to COVID-19-related customers, but also customers with non-COVID-19 applications who built up stock in vials to mitigate supply chain uncertainty and manage long lead times at the height of the pandemic. We believe this is a temporary imbalance of supply and demand across the industry.
Fiscal Year 2023 Challenges
For the year ended December 31, 2023, we confronted a number of challenges linked, in part, to the impact of the COVID-19 pandemic and, in part, to the conflict between Russia and Ukraine, and due to the conflicts in the Middle
72
East. The military actions undertaken by Russian military forces against Ukraine resulted in the imposition of financial and economic sanctions by the European Union, the U.S., the United Kingdom and certain other countries and organizations against officials, individuals, regions, and industries in Russia and Belarus. As a result, primarily of the Russian-Ukrainian conflict, in 2022 gas prices fluctuated dramatically and affected Group margins. However, Stevanato Group has not faced any difficulties in accessing natural gas for its operations to date. For the year ended December 31, 2023, gas prices have stabilized, and we continue to monitor the situation going forward.
The increased demand for electrical components, coupled with COVID-19 lockdowns in China, has impacted global supply chains, which were already under strain. While this has caused a temporary disruption in the supply of the manufacturing parts that we use, our ability to source from multiple suppliers, order materials in advance, and keep excess raw materials and inventory on hand, has limited the impact on our business of these supply chain challenges. In 2023 the elongated lead times for electronic components, coupled with high demand, has caused some project delays which has lengthened our delivery times for Engineering equipment.
As a consequence of the above, there has been a trend of increasing inflation, to which the U.S. Federal Reserve and other central banks in various countries have responded by raising interest rates to restrictive levels in 2023. Even if prices may remain volatile for some time, in 2023 there has been an easing of inflation pressures.
General global economic downturns and macroeconomic trends, including heightened inflation, capital market volatility, interest rate and currency rate fluctuations, and an economic slowdown or recession, may result in unfavorable conditions that could negatively affect demand for our products and exacerbate some of the other risks that affect our business, financial condition and results of operations. Furthermore, currency exchange rates have been especially volatile in the recent past, and these currency fluctuations have affected, and may continue to affect, the reported value of our assets and liabilities, as well as cash flows.
Maximizing Efficiency in Our Production Processes
Our production costs depend on our ability to maximize efficiency in our production processes. In 2018, we launched “SG Steps”, an operational excellence program aimed, among other things, at continuously improving the efficiency of our processes. Due to the integrated nature of our business, the “SG Steps” program resulted in an increase in the overall effectiveness of the equipment in our facilities, improved quality and efficiency in our global production processes and reduced delivery time, returns from customers, production scraps and waste. Our ability to maintain low production costs and, as a consequence, increase our profitability, will depend, in part, on how successful we are in further maximizing the efficiency of our processes.
Research and Development Expenses
In 2023, our research and development expenses amounted to 3.3% of our revenue, compared to 3.5% in 2022. Expenses in research and new product development are a strategic enabler for our future growth and we expect to continue to make substantial investments in this area in coming years. Through continued spending in our research and development programs, we intend to drive revenue and profit growth through processes that will improve innovation and quality of our existing products, and facilitate the shift towards “high-value” products, services and solutions.
Our ability to leverage our recent investments in research and new product development is critical to our future performance. We will continue to develop (i) containment solutions for innovative biologic drugs, including GLP1s, mRNA applications, monoclonal antibodies, cell and gene therapies, and (ii) a sustainable pipeline of patient-centric drug delivery systems that support the transition of therapy from hospital to home care and facilitate patient self-administration.
In our core drug containment solutions business, the development of new products will be targeted at maintaining the stability, potency and purity of our customers’ products prior to administration. New therapies for diabetes, obesity, cancer and autoimmune diseases are based on large, complex biological molecules that are extremely sensitive to their storage environment such as mRNA applications. In the area of drug delivery systems, we will be targeting the development of easy-to-use, accurate, reliable self-injection systems for complex pharmaceutical and biotechnology products. We have developed a portfolio of devices for this market that can be used off-the-shelf or tailored to the specific needs of the customer.
73
We will continue developing new drug delivery systems based on three pillars: patient centricity, sustainability and digitalization, all of which are core capabilities to meet our customer’s need for connected health devices.
We apply a rigorous “stage & gate” development process to de-risk our development projects and reduce total development costs. Development timelines for new drug delivery devices typically fall into the range of four to five years to reach the start of initial production.
Components of Our Results of Operations
The following discussion sets forth certain components of our statements of operations as well as factors that impact those items.
Results discussed in this section of the annual report are consolidated according to International Financial Reporting Standards as issued by International Accounting Standards Board and therefore does not include Company’s inter-segment items other than where we specifically note otherwise.
Revenue and Segment Reporting
Our business operations are divided into two segments:
Revenue recognized in the years ended December 31, 2023 and 2022, amounted to €1,085.4 million and €983.7 million, respectively.
For each of the years ended December 31, 2023 and 2022, our Biopharmaceutical and Diagnostic Solutions segment represented 81% of revenue while our Engineering segment represented 19% of revenue, respectively.
The following tables set forth the results of our business operations for the aforementioned segments, which include inter-segment items, and the reconciliation with the consolidated figures, for the year ended December 31, 2023, and 2022.
Revenue for each segment is divided into "External Customers", representing revenue from third parties' sales, and "Inter-Segment", representing the revenue from the sales generated from the transactions with other segments, and is then reconciled with the Consolidated Revenue which does not include inter-segment items.
Gross Profit margin is calculated by dividing Gross Profit for a period by total revenue for the same period. Operating Profit margin is calculated by dividing Operating Profit for a period by total revenue for the same period. Gross Profit margin and Operating profit margin for both Biopharmaceutical and Diagnostic Solutions segment and Engineering segment include the effect of inter-segment transactions.
74
|
|
For the year ended December 31, 2023 |
||||||
|
|
Biopharmaceutical |
|
Engineering |
|
Adjustments, |
|
Consolidated |
|
|
|
|
|
|
|
|
|
External Customers |
|
879.3 |
|
206.1 |
|
— |
|
1,085.4 |
Inter-Segment |
|
2.0 |
|
166.7 |
|
(168.7) |
|
— |
Revenue |
|
881.3 |
|
372.8 |
|
(168.7) |
|
1,085.4 |
|
|
|
|
|
|
|
|
|
Gross Profit |
|
290.2 |
|
78.3 |
|
(28.6) |
|
339.9 |
Gross Profit Margin |
|
32.9% |
|
21.0% |
|
|
|
31.3% |
|
|
|
|
|
|
|
|
|
Operating Profit |
|
187.6 |
|
53.6 |
|
(40.5) |
|
200.7 |
Operating Profit Margin |
|
21.3% |
|
14.4% |
|
|
|
18.5% |
|
|
For the year ended December 31, 2022 |
||||||
|
|
Biopharmaceutical |
|
Engineering |
|
Adjustments, |
|
Consolidated |
|
|
|
|
|
|
|
|
|
External Customers |
|
799.7 |
|
184.0 |
|
— |
|
983.7 |
Inter-Segment |
|
1.5 |
|
115.5 |
|
(117.0) |
|
— |
Revenue |
|
801.2 |
|
299.5 |
|
(117.0) |
|
983.7 |
|
|
|
|
|
|
|
|
|
Gross Profit |
|
274.9 |
|
64.7 |
|
(19.7) |
|
319.9 |
Gross Profit Margin |
|
34.3% |
|
21.6% |
|
|
|
32.5% |
|
|
|
|
|
|
|
|
|
Operating Profit |
|
182.6 |
|
41.3 |
|
(31.4) |
|
192.5 |
Operating Profit Margin |
|
22.8% |
|
13.8% |
|
|
|
19.6% |
75
Results of Operations
Year ended December 31, 2023 versus year ended December 31, 2022
The following table sets forth our results of operations for the years ended December 31, 2023 and 2022.
|
|
(Amounts in € millions, except as indicated otherwise) |
||||||||||
|
|
For the year ended December 31, |
|
Change |
|
Change |
||||||
|
|
2023 |
|
% of revenue |
|
2022 |
|
% of revenue |
|
€ |
|
% |
Revenue |
|
1,085.4 |
|
100.0% |
|
983.7 |
|
100.0% |
|
101.7 |
|
10.3% |
Costs of Sales |
|
745.5 |
|
68.7% |
|
663.9 |
|
67.5% |
|
81.6 |
|
12.3% |
Gross Profit |
|
339.9 |
|
31.3% |
|
319.8 |
|
32.5% |
|
20.1 |
|
6.3% |
Other Operating Income |
|
10.4 |
|
1.0% |
|
18.8 |
|
1.9% |
|
(8.4) |
|
(44.7)% |
Selling and Marketing Expenses |
|
25.0 |
|
2.3% |
|
26.1 |
|
2.7% |
|
(1.1) |
|
(4.2)% |
Research and Development Expenses |
|
35.7 |
|
3.3% |
|
34.4 |
|
3.5% |
|
1.3 |
|
3.7% |
General and Administrative Expenses |
|
88.9 |
|
8.2% |
|
85.7 |
|
8.7% |
|
3.2 |
|
3.7% |
Operating Profit |
|
200.7 |
|
18.5% |
|
192.4 |
|
19.6% |
|
8.3 |
|
4.3% |
Finance Income |
|
20.3 |
|
1.9% |
|
25.0 |
|
2.5% |
|
(4.7) |
|
(19.2)% |
Finance Expense |
|
31.4 |
|
2.9% |
|
29.8 |
|
3.0% |
|
1.6 |
|
5.3% |
Profit Before Tax |
|
189.6 |
|
17.5% |
|
187.6 |
|
19.1% |
|
2.0 |
|
1.0% |
Income Taxes |
|
43.9 |
|
4.0% |
|
44.6 |
|
4.5% |
|
(0.7) |
|
(1.7)% |
Net Profit |
|
145.7 |
|
13.4% |
|
143.0 |
|
14.5% |
|
2.7 |
|
1.9% |
Revenue
Revenue increased by €101.7 million, or 10.3%, to €1,085.4 million for the year ended December 31, 2023, compared to €983.7 million for the year ended December 31, 2022, driven by growth in both segments, higher volumes and the mix shift to high value solutions. For the year ended December 31, 2023 we estimate that COVID-19 related revenue was approximately 1.6% of our total revenue, compared to 11.2% for the year ended December 31, 2022. Revenue growth on constant currency basis was 11.2% for the year ended December 31, 2023.
Biopharmaceutical and Diagnostic Solutions
|
|
(Amounts in € million, except as indicated otherwise) |
|
|||||||||||||
|
|
For the year ended |
|
|
Change |
|
|
Change |
|
|||||||
|
|
2023 |
|
|
2022 |
|
|
€ |
|
|
% |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Type of goods or service |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Revenue from high-value solutions |
|
|
366.4 |
|
|
|
293.2 |
|
|
|
73.2 |
|
|
|
25.0 |
% |
Revenue from other containment and delivery solutions |
|
|
512.9 |
|
|
|
506.4 |
|
|
|
6.5 |
|
|
|
1.3 |
% |
Total Revenue from |
|
|
879.3 |
|
|
|
799.7 |
|
|
|
79.6 |
|
|
|
10.0 |
% |
Revenue generated by the Biopharmaceutical and Diagnostic Solutions segment increased by €79.6 million, or 10.0%, to €879.3 million for the year ended December 31, 2023 compared to €799.7 million in the year ended December 31, 2022. For the year-ended December 31, 2023, we estimate revenue related to COVID-19 decreased by approximately €93.3 million or 84.4% to €17.2 million and represented 2.0% of the segment revenue compared to approximately €110.5 million, or 13.8% of the segment revenue for the year ended December 31, 2022.
Revenue from our premium priced, high-value solutions increased by €73.2 million, or 25.0%, to €366.4 million for the year ended December 31, 2023, compared to €293.2 million for the year ended December 31, 2022, reflecting
76
increased demand for high performance drug containment products. Revenue generated by other containment and delivery solutions increased by €6.5 million, or 1.3%, to €512.9 million for the year ended December 31, 2023, compared to €506.4 million for the year ended December 31, 2022.
Throughout fiscal year 2023, the segment experienced lower volumes and revenue attributable to glass vials and in-vitro diagnostics, as customers continue to work down higher inventories resulting from COVID-19. This was offset by higher demand and revenue related to other products, such as syringes, within the Biopharmaceutical and Diagnostic Solutions segment.
On a constant currency basis, revenue generated by high-value solutions increased by €77.6 million, or 26.5%, for the year ended December 31, 2023, compared to €293.2 million for the year ended December 31, 2022, while revenue generated by other containment and delivery solutions increased by €10.2 million, or 2.0%, for the year ended December 31, 2023 compared to €506.4 million for the year ended December 31, 2022.
Engineering
Revenue generated by the Engineering segment, increased by €22.0 million, or 12.0%, to €206.1 million for the year ended December 31, 2023 compared to €184.0 million for the year ended December 31, 2022. The growth was driven by higher sales in visual inspection systems, assembly and packaging machines and after-sales support services.
We do not consider any of the revenue in our Engineering segment to be attributable to COVID-19 since: (i) we cannot accurately determine the end users of our products; and (ii) most of our products have life cycles of 10 years or more and, therefore, bear a reasonably likely chance of being used for purposes other than COVID-19 related.
Revenue Breakdown by Region
The following tables present revenue by geographical markets for the year ended December 31, 2023, and 2022. Revenue by geographical markets is based on the end customer location. The reported geographical markets are EMEA (Europe, Middle East, Africa), North America (United States, Canada, Mexico), South America and APAC (Asia Pacific).
|
|
(Amounts in € million, except as indicated otherwise) |
|
|||||||||||||||||||||
|
|
For the year ended December 31, |
|
|
Change |
|
|
Change |
|
|||||||||||||||
|
|
2023 |
|
|
% on Revenue |
|
|
2022 |
|
|
% on Revenue |
|
|
€ |
|
|
% |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Geographical markets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
EMEA |
|
|
634.1 |
|
|
|
58.4 |
% |
|
|
599.7 |
|
|
|
61.0 |
% |
|
|
34.4 |
|
|
|
5.7 |
% |
APAC |
|
|
102.9 |
|
|
|
9.5 |
% |
|
|
100.3 |
|
|
|
10.2 |
% |
|
|
2.6 |
|
|
|
2.6 |
% |
North America |
|
|
317.5 |
|
|
|
29.3 |
% |
|
|
250.8 |
|
|
|
25.5 |
% |
|
|
66.7 |
|
|
|
26.6 |
% |
South America |
|
|
30.9 |
|
|
|
2.8 |
% |
|
|
32.9 |
|
|
|
3.3 |
% |
|
|
(2.0 |
) |
|
|
(6.1 |
)% |
Total Revenue |
|
|
1,085.4 |
|
|
|
100.0 |
% |
|
|
983.7 |
|
|
|
100.0 |
% |
|
|
101.7 |
|
|
|
10.3 |
% |
Cost of Sales
Cost of sales increased by €81.6 million, or 12.3%, to €745.5 million for the year ended December 31, 2023 compared to €663.9 million for the year ended December 31, 2022, mainly due to the growth in sales volumes and of the increase in industrial costs, such as materials and labor, tied to the start-up of our new manufacturing plants. The increase in cost of sales also resulted from an increase in industrial depreciation due to the availability for use of the machinery installed in the previous periods to increase the production capacity, particularly for high-value solutions.
For the year ended December 31, 2023, cost of sales included €10.6 million of start-up costs principally related to the new facilities in Indiana, U.S., and Latina, Italy. These costs are primarily related to labor costs incurred prior to the ongoing start-up of commercial operation that are associated with the training and travel of personnel who are employed in the production of our products, which require specific knowledge.
77
For the year ended December 31, 2022, cost of sales included €3.3 million of start-up costs principally related to the new facilities in Indiana, U.S., and Latina, Italy.
As a percentage of revenue, cost of sales was 68.7% for the year ended December 31, 2023 compared to 67.5% for the year ended December 31, 2022.
Gross Profit
For the year ended December 31, 2023, gross profit increased by €20.1 million, or 6.3%, to €339.9 million for the year ended December 31, 2023 compared to €319.8 million for the year ended December 31, 2022. Gross profit margin decreased to 31.3% for the year ended December 31, 2023 compared to 32.5% for the year ended December 31, 2022, due to the decrease in gross profit margin from Biopharmaceutical and Diagnostic Solution segment and to a lesser extent to the decrease in gross profit margin from Engineering segment.
For the year ended December 31, 2023, gross profit margin for the Biopharmaceutical and Diagnostic Solution segment was 32.9% compared to 34.3% for the year ended December 31, 2022. The decrease in gross profit margin was mainly due to lower EZ-Fill® vial volumes, industrial costs tied to the start-up of our new manufacturing plants, higher depreciation, and currency translation. We currently expect that margins will continue to be tempered in 2024 by ramp-up inefficiencies and higher depreciation as we grow volumes in our new manufacturing facilities and gain better leverage on our fixed costs.
Engineering segment gross profit margin decreased to 21.0% for the year ended December 31, 2023, compared to 21.6% for the year ended December 31, 2022. The decrease in gross profit margin was mainly driven by lower marginality on specific projects in process. In the second half of 2023, the combination of strong demand and supply chain volatility placed stress on our engineering resources, and this resulted in some delays.
Other Operating Income
For the year ended December 31, 2023, other income decreased by €8.4 million, or 44.7%, to €10.4 million, compared to €18.8 million for the year ended December 31, 2022, which included approximately €7.6 million income related to a compensation for cancellation of an unfulfilled contract.
Selling and Marketing Expenses
Selling and marketing expenses decreased by €1.1 million, or 4.2%, to €25.0 million for the year ended December 31, 2023 compared to €26.1 million for the year ended December 31, 2022. Selling and marketing expenses are lower compared to the year ended December 31, 2022 due to cost containment initiatives and a lower cash bonus accrual tied to achievement thresholds under the Group's performance-based management bonus program as well as lower share-based compensation expense accrual following the introduction of the 2023-2027 incentive plans. The decrease was partially offset by an increase in the accruals of bad debt provision (€0.7 million accrual for the year ended December 31, 2023 compared to €0.8 million release for the year ended December 31, 2022).
As a percentage of revenue, selling and marketing expenses was 2.3% for the year ended December 31, 2023 compared to 2.7% for the year ended December 31, 2022.
Research and Development Expenses
Research and development expenses increased by €1.3 million, or 3.7%, to €35.7 million for the year ended December 31, 2023, compared to €34.4 million for the year ended December 31, 2022. Such expenses include costs for research and development activities to support the innovation of our product range and components as well as amortization and depreciation for €4.8 million for the year ended December 31, 2023 (€3.5 million for the year ended December 31, 2022). The increase in research and development expenses is primarily due to an increase in personnel expenses and higher depreciation and amortization partially offset by containment of external project costs.
As a percentage of revenue, research and development expenses was 3.3% for the year ended December 31, 2023 compared to 3.5% for the year ended December 31, 2022.
78
General and Administrative Expenses
General and administrative expenses increased by €3.2 million, or 3.7%, to €88.9 million for the year ended December 31, 2023, compared to €85.7 million in the year ended December 31, 2022. These expenses include depreciation and amortization of €8.3 million (compared to €7.1 million in 2022), of which amortization of fair value adjustments from purchase price allocations amounted to €1.0 million (€1.0 million in 2022). The increase in general and administrative expenses was mainly attributable to higher IT related costs, such as licenses, and to higher depreciation and amortization. This was partially offset by a lower cash bonus accrual tied to achievement thresholds under the Group's performance-based management bonus program as well as lower share-based compensation expense accrual following the introduction of the 2023-2027 incentive plans.
For the year ended December 31, 2023, general and administrative expenses included €1.4 million of start-up costs principally related to the new facilities in Indiana, U.S., and Latina, Italy and €0.3 million of restructuring and related charges.
For the year ended December 31, 2022 general and administrative expenses included €2.9 million of start-up costs principally related to the new facilities in Indiana, U.S., and Latina, Italy.
As a percentage of revenue, general and administrative expenses was 8.2% for the year ended December 31, 2023 compared to 8.7% for the year ended December 31, 2022.
Operating Profit
For the year ended December 31, 2023, operating profit increased by €8.3 million, or 4.3%, to €200.7 million for the year ended December 31, 2023, compared to €192.4 million for the year ended December 31, 2022. Operating profit margin for the year ended December 31, 2023 decreased to 18.5% compared to 19.6% for the year ended December 31, 2022.
For the year ended December 31, 2023, the operating profit margin for the Biopharmaceutical and Diagnostic Solution segment was 21.3%, compared to 22.8% for the year ended December 31, 2022. The decrease in operating profit margin was primarily due to the reduction in gross profit margin and other operating income, partially offset by better absorption of SG&A expenses.
For the year ended December 31, 2023, Engineering operating profit margin was 14.4%, compared to 13.8% for the year ended December 31, 2022. The increase in operating profit margin was primarily due to an increased absorption of SG&A expenses.
Net Finance Expenses
Finance expenses, net of finance income, increased by €6.3 million to a net expense balance of €11.1 million for the year ended December 31, 2023, from a net expense balance of €4.8 million for the year ended December 31, 2022. The net change was mainly due to unrealized foreign exchange losses as a result of the strengthening of some currencies, such as the EUR and MXN, against the USD and, to a lesser extent, to the increased interest expenses, primarily related to the use of short-term loan facilities.
Profit Before Tax
Profit before taxes increased by €2.0 million, or 1.0%, to €189.6 million for the year ended December 31, 2023, compared to €187.6 million for the year ended December 31, 2022.
Income Taxes
Income taxes decreased by €0.7 million, or 1.7%, to €43.9 million for the year ended December 31, 2023, compared to €44.6 million for the year ended December 31, 2022 as described below. The effective tax rate for the year ended December 31, 2023, decreased to 23.1% compared to 23.8% for the year ended December 31, 2022.
79
|
|
(Amounts in € millions, except as indicated otherwise) |
||||
|
|
For the year |
|
Change |
||
|
|
2023 |
|
2022 |
|
€ |
Current Income Tax |
|
|
|
|
|
|
Current Taxes |
|
60.7 |
|
57.6 |
|
3.1 |
Deferred Taxes |
|
|
|
|
|
|
Deferred Taxes |
|
(16.8) |
|
(13.0) |
|
(3.8) |
Income Tax Expenses reported in the statement of profit or loss |
|
43.9 |
|
44.6 |
|
(0.7) |
Current Taxes
Current taxes increased by €3.1 million, or 5.3%, to €60.7 million for the year ended December 31, 2023, compared to €57.6 million for the year ended December 31, 2022. This increase was primarily due to higher taxable income in the Italian Group’s legal entities for the year ended December 31, 2023.
Deferred Taxes
For the year ended December 31, 2023, we recorded a deferred taxes benefit of €16.8 million, compared to the €13.0 million tax benefit for the year ended December 31, 2022. For the year ended December 31, 2023 the tax benefit was mainly connected to (i) the deferred tax assets accrual on land grant in the legal entity Ompi of America, (ii) the deferred tax assets booked upon consolidated elimination of revenues related to intercompany sales of fixed assets and (iii) to deferred tax assets recorded on tax losses carry forward.
Net Profit
Net profit increased by €2.7 million, or 1.9%, to €145.7 million (or €0.55 of Diluted EPS or €0.58 of Adjusted Diluted EPS) for the year ended December 31, 2023, compared to €143.0 million (or €0.54 of Diluted EPS or €0.56 of Adjusted Diluted EPS) for the year ended December 31, 2022. For details on "Adjusted Diluted EPS" see "Key Indicators of Performance and Financial Condition - Adjusted Operating Profit, Adjusted Operating Profit Margin, Adjusted Net Profit and Adjusted Diluted EPS" below.
Year ended December 31, 2022 compared to the year ended December 31, 2021
For a discussion of our results for the year ended December 31, 2022 compared to the year ended December 31, 2021, please see “Item 5. Operating and Financial Review and Prospects - A. Operating Result - Results of Operations - Three months and year ended December 31, 2022, versus three months and year ended December 31, 2021” contained in our annual report on Form 20-F for the year ended December 31, 2022, filed with the SEC on March 2, 2023.
B. LIQUIDITY AND CAPITAL RESOURCES
We finance our operations mainly through cash generated by our operating activities and debt financing. Our primary requirements for liquidity and capital are to finance capital expenditures, working capital (which is the difference of current assets and current liabilities—net of current financial assets, current financial liabilities, cash and cash equivalents), and general corporate purposes.
Our primary sources of liquidity are our cash and cash equivalents, short-term loan facilities, and medium and long-term loans from a number of financial institutions, as described below, and the equity markets. At December 31, 2023, we had cash and cash equivalents of €69.6 million (compared to €228.7 million in 2022) and other current financial assets (other than derivatives) of €0.7 million (compared to €27.9 million in 2022). Our cash and cash equivalents primarily consist of cash at bank and highly liquid investments, such as short-term deposits, which are unrestricted from withdrawal or use, or which have original maturities of three months or less when purchased. We believe that our total available liquidity (defined as cash and cash equivalents, plus undrawn committed credit lines),
80
in addition to funds that will be generated from operating activities, and the potential access to additional capital through the equity markets or through additional loan or debt agreements, will enable us to satisfy the requirements of our investing activities and working capital needs for at least the next 12 months and ensure an appropriate level of operating and strategic flexibility.
In January and March 2024, we secured two loans totaling €80.0 million to support our ongoing capital investments in growth platforms. The first loan agreement was financed by BPER Banca for €30.0 million and the second loan for €50.0 million was financed by Intesa Sanpaolo. Both financings have a five-year tenor, with two years of interest-only payments and three years of amortizing period. The loans are intended to finance capacity expansion to increase production, primarily for high-value solutions to meet increased demand.
Our total current liabilities were €574.4 million as of December 31, 2023 (compared to €462.5 million as of December 31, 2022), which primarily includes €277.8 million trade payables, €22.3 million contract liabilities, €22.9 million advances from customers, €137.4 million financial liabilities, €30.8 million tax payables, €5.8 million lease liabilities, €1.1 million current provision for warranty, and €76.2 million other liabilities mainly relating to payables to personnel and social security institutions as well as allowance for future expected customer returns.
Financing activities
We employ a disciplined approach in managing our working capital and balance sheet to support our business and operations.
Pricoa Private Placement
On April 16, 2020 we entered into a note purchase and private shelf agreement with PGIM, Inc. and certain of its affiliates (the “Note Purchase Agreement”), pursuant to which, for a period of three years following the date of the agreement (unless terminated earlier) we had the right to issue, and PGIM, Inc. or certain of its affiliates had the right to purchase, up to $69.5 million of our notes. Pursuant to the Note Purchase Agreement, on the same date, we issued €50.0 million of our Senior Notes, Series A, due April 16, 2028 to PGIM, Inc. (the “Notes”), with an interest rate of 1.4%. Repayment of the Notes is required to be made in two tranches, €25.0 million on April 16, 2027, and the reminder at the expiration of the notes.
Pursuant to the Note Purchase Agreement, Nuova Ompi S.r.l. provided to PGIM, Inc. and its affiliates a subsidiary guarantee, guaranteeing the repayment of the notes.
The Note Purchase Agreement imposes certain covenants on us, including: (i) the notes must always rank at least pari passu with all other unsecured and unsubordinated indebtedness of the company and the guarantor; (ii) any covenant included in a different financing agreement which is more favorable to the lenders must apply to the Note Purchase Agreement, as well; (iii) the aggregate EBITDA of the company and the guarantor must always be at least equal to a certain percentage of the EBITDA of our group; (iv) no merger or consolidation for any guarantor unless expressly permitted by the Note Purchase Agreement; (v) no dealings with sanctioned entities; (vi) the ratio of consolidated net debt to consolidated EBITDA not to be greater than 3.50 to 1.00 with an increase of up to 4.0x once; (vii) consolidated net debt to equity not to be greater than 2 to 1; (viii) no liens in excess of a certain amount except for, among others, (a) existing ones, (b) tax liens, (c) liens in the ordinary course of business, (d) judgment liens; (ix) no sale of assets in excess of a certain amount; (x) no subsidiary indebtedness beyond a certain basket; and (xi) no segregation of assets under Italian law.
As at December 31, 2023 and 2022, all financial covenants are complied with.
Additional Medium and Long-Term Loan Facilities
As of December 31, 2023, we had medium and long-term loan facilities totaling €243.9 million fully drawn down.
The total outstanding amount was raised between 2017, 2019 and 2023. Approximately €59.5 million outstanding as of December 31, 2023 were raised in 2019 from four banks. The average term is 2.3 years. The average all-in fixed interest rate, inclusive of hedging and upfront fees, is 1.4%. These loan agreements impose certain covenants on us, including: (i) not to exceed certain consolidated net debt to consolidated EBITDA ratios (not greater than 4.0 to 1.0
81
in three of the loan agreements and not greater than 3.5 to 1.0, at 4.0x, in the remaining agreements); (ii) to maintain a consolidated net debt to equity ratio equal to or lower than 2 to 1 and at least amounting to €200 million; (iii) not to sell assets having a value, or to grant liens or loans to third parties, exceeding certain amounts; (iv) to ensure that the loans always rank at least pari passu with other debt of the company; (v) not to segregate assets (as defined under Italian law); and (vi) not to distribute dividends or reserves nor to carry out extraordinary transactions resulting in the breach of financial covenants.
As at December 31, 2023 and 2022, all financial covenants are complied with.
Approximately €22.2 million outstanding as of December 31, 2023 were raised in 2017 and 2018. The average term is 1.7 years. These loans include covenants consistent with those described for the 2019 loans. The average all-in fixed interest rate, inclusive of hedging and upfront fees, is 1.2%.
On January 16, 2023 and February 28, 2023 we entered into two term loan contracts, totaling €130.0 million, to support production capacity expansion in the U.S. and Italy. The first loan agreement was financed through BNL (Group BNP Paribas) for €70.0 million and the second loan for €60.0 million was financed through Cassa Depositi e Prestiti. Both financings are at floating rates and have a five-year tenor with two years of interest-only payments and three years of amortizing period with quarterly repayment of the installments at constant principal portion. Both loans are “ESG-linked” with potential price improvement linked to the yearly successful achievement of two ESG KPIs. As of December 31, 2023, both loans have been fully drawn down. These loans include covenants consistent with those described for the 2019 loans.
On November 30, 2023 and December 19, 2023, we entered into two term loan agreements with Monte Dei Paschi di Siena, totaling €30.0 million, to support production capacity expansion. Both financings, which have been fully drawn down, are at floating rates and have a five-year tenor with two years of interest-only payments and three years of amortizing period with quarterly repayment of the installments at constant principal portion. These loans include covenants consistent with those described for the 2019 loans.
Short-Term Loan Facilities
As of December 31, 2023, we had short-term facilities totaling €143.3 million in available principal, of which we had drawn down €84.0 million. Of these €84.0 million, €26.0 million are denominated in Danish kroner for a countervalue of DKK 198.8 million.
Capital Expenditures
During the fiscal year ended December 31, 2023, capital expenditures amounted to €453.3 million. Capital expenditure for growth and capacity expansion (defined as all investments related to existing capacity increase, i.e. new industrial lines, new buildings, warehouse/production unit expansion) were €417.3 million, which included (i) €369.4 million for new EZ-Fill® production lines and related buildings expansion, principally in Fishers, U.S., (€216.9 million) and in Latina, Italy, (€120.5 million), (ii) €24.8 million for the completion of our glass containment solutions capacity expansion, (iii) €14.1 million for new machinery for high precision plastic injection molding and assembly for container in vitro diagnostic solutions and (iv) €8.9 million dedicated mainly to Engineering capacity expansion, ITC capacity increase and molds.
In the U.S., construction of the new facility continues to progress. The plant launched customer validation and expects to start commercial operations in 2024. In Italy, the new facility in Piombino Dese started commercial production in early 2023 while in Latina the Group launched commercial production in the fourth quarter of 2023. In 2023, the Group decided to delay the timing of our investment in China in order to focus efforts and resources on the ramp up of its manufacturing facilities in the U.S. and Italy where demand outpaced initial expectations.
As at December 31, 2023 committed orders related to the ongoing investments equaled approximately €136.5 million, net of the expected contribution from the U.S. government’s Biomedical Advanced Research and Development Authority (BARDA).
82
Capital expenditures for maintenance, increasing quality, improving our IT systems, improving efficiency of our production processes and improving safety of our plants and production sites amounted to €25.0 million and €11.0 million for research and development, including laboratory equipment, molds and other related equipment.
Cash Flow
Year ended December 31, 2023 versus year ended December 31, 2022
The following table presents the summary consolidated cash flow information for the years ended December 31, 2023 and 2022.
|
|
(Amounts in € millions, except as indicated otherwise) |
||||
|
|
For the year |
|
Change |
||
|
|
2023 |
|
2022 |
|
€ |
Cash flows from operating activities |
|
105.2 |
|
103.3 |
|
1.9 |
Cash flows used in investing activities |
|
(421.2) |
|
(243.0) |
|
(178.2) |
Cash flows from / (used in) financing activities |
|
158.0 |
|
(44.5) |
|
202.5 |
Net change in cash and cash equivalents |
|
(158.0) |
|
(184.2) |
|
26.2 |
Cash generated from operating activities
Net cash generated from operating activities was €105.2 million for the year ended December 31, 2023 (compared to €103.3 million for the year ended December 31, 2022). For the year ended December 31, 2023 the net cash generated from operating activities was primarily the result of (i) profit before taxes of €189.6 million adjusted for €78.5 million of depreciation and amortization expense, €3.7 million of net finance expense and €0.5 million of gains from the disposal of fixed assets, (ii) €2.4 million from change in provisions, including the allowance for bad and doubtful debts, and employee benefits, (iii) €13.0 million net other non-cash expenses and (iv) €105.0 million cash generated from the change in trade payables, contract liabilities, advances and other liabilities. These cash inflows were partially offset by (i) €113.6 million cash absorbed from the net change in inventories and contract assets, (ii) €93.9 million from the net change in trade receivables e and other assets, (iii) €76.8 million income tax paid and (iv) €2.2 million net finance interests paid.
Net cash generated from operating activities was €103.3 million for the year ended December 31, 2022. For the year ended December 31, 2022 the EBITDA generation of €257.3 million was partially offset by (i) €117.7 million cash absorbed from the net change in working capital, deriving from the growth and mainly due to inventories increase to secure supply chain, (ii) €9.0 million cash absorbed from the change in other provision and employees benefits and (iii) €25.8 million in income tax paid.
Cash used in investing activities
Net cash used in investing activities was €421.2 million for the year ended December 31, 2023 (compared to €243.0 million cash used in investing activities for the year ended December 31, 2022), as we continued to execute our strategic investments in capacity expansion for high value solutions to meet customer demand. For the year ended December 31, 2023 net cash used in purchasing property and equipment as well as intangible assets amounted to €441.9 million net of the advance payment of approximately €22.4 million received from the U.S. Biomedical Advanced Research and Development Authority (BARDA) which reflects a partial payment for installing machinery in Fishers, Indiana, to help strengthen domestic capabilities in the U.S. for national defense readiness and preparedness programs for current and future public health emergencies.
For the year ended December 31, 2023 the net cash spent to acquire Perugini S.r.l. was €3.6 million while the cash used for investing in other financial assets amounted to €4.2 million.
These cash outflows were partially offset by the proceeds from the life insurance policies redemption for €27.9 million.
83
Net cash used in investing activities was €243.0 million for the year ended December 31, 2022 (compared to €96.4 million cash used in investing activities for the year ended December 31, 2021), consisting mainly of the purchase of property and equipment to increase our production capacity for both our EZ-Fill® product portfolio and our bulk business. For the year ended December 31, 2022 net cash used in investing activities also included (i) the advance payment of approximately €13.9 million received from the BARDA for installing machinery in Fishers and (ii) the advance payment of approximately €2.3 million received from the city of Fishers for hard costs at site. We also invested in R&D and in strengthening the security of our IT systems.
Cash used in and generated from/ (used in) financing activities
Net cash flows generated from financing activities was €158.0 million for the year ended December 31, 2023 (compared to €44.5 million generated from financing activities for the year ended December 31, 2022). For the year ended December 31, 2023, the net cash generated from financing activities was primarily related to the proceeds from borrowings for €247.5 million. These cash inflows were partially offset by € 69.0 million repayments of borrowings, by €5.9 million payment of the principal portion of lease liabilities, by €14.3 million dividends paid and by €0.3 million for the acquisition of non-controlling interests in Ompi of Japan Co. Ltd.
Net cash flows used in financing activities was €44.5 million for the year ended December 31, 2022 (compared to €254.8 million generated from financing activities for the year ended December 31, 2021). Cash raised from new borrowings amounted to €13.2 million while the loan repayments amounted to €37.6 million. Dividends distributed amounted to €13.5 million, payment of the principal portion of lease liabilities was €6.6 million.
Net change in cash and cash equivalents
The net change in cash and cash equivalents was a reduction of €158.0 million for the year ended December 31, 2023, compared to a reduction of €184.2 million for the year ended December 31, 2022.
Off Balance Sheet Arrangements
Off-balance sheet arrangements may be summarized as follows:
|
|
(Amounts in € millions) |
|
|||||
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
Guarantees |
|
|
128.1 |
|
|
|
112.4 |
|
of which secured |
|
|
4.7 |
|
|
|
4.7 |
|
Total Guarantees |
|
|
128.1 |
|
|
|
112.4 |
|
At December 31, 2023, we issued guarantees to third parties for €128.1 million in the ordinary course of business. Such amount includes advance payment and performance bonds as well as suretyships and letters of comfort to financial institutions on outstanding short-term facilities in favor of foreign subsidiaries; some of which include floating charges for €4.7 million.
84
Tabular Disclosure of Contractual Obligations and Commitments
The following table summarizes payments due under our contractual obligations and commitments at December 31, 2023:
|
|
(Amounts in € millions, except as indicated otherwise) |
|
|||||||||||||||||
|
|
Due within one year |
|
|
Due between two and three years |
|
|
Due between four and five years |
|
|
Due beyond five years |
|
|
Total |
|
|||||
Borrowings (1) |
|
|
135.6 |
|
|
|
117.6 |
|
|
|
74.7 |
|
|
|
— |
|
|
|
327.9 |
|
Notes |
|
|
0.1 |
|
|
|
0.1 |
|
|
|
49.5 |
|
|
|
— |
|
|
|
49.7 |
|
Lease liabilities (2) |
|
|
5.8 |
|
|
|
6.5 |
|
|
|
2.2 |
|
|
|
4.4 |
|
|
|
18.9 |
|
Other financial liabilities |
|
|
0.2 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
0.2 |
|
Trade payables |
|
|
277.8 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
277.8 |
|
Tax payables |
|
|
30.8 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
30.8 |
|
Other liabilities (3) |
|
|
63.4 |
|
|
|
1.7 |
|
|
|
— |
|
|
|
— |
|
|
|
65.1 |
|
Employee Benefits |
|
|
0.1 |
|
|
|
0.2 |
|
|
|
— |
|
|
|
7.1 |
|
|
|
7.4 |
|
Total liabilities |
|
|
513.8 |
|
|
|
126.1 |
|
|
|
126.4 |
|
|
|
11.5 |
|
|
|
777.9 |
|
Key Indicators of Performance and Financial Condition
Non-GAAP Financial Measures
We monitor and evaluate our operating and financial performance using several non-GAAP financial measures, including: Constant Currency Revenue, EBITDA, Adjusted EBITDA, Adjusted EBITDA Margin, Adjusted Operating Profit, Adjusted Operating Profit Margin, Adjusted Income Taxes, Adjusted Net Profit, Adjusted Diluted EPS, CAPEX, Free Cash Flow, Net (Debt)/ Cash and Capital Employed. We believe that these non-GAAP financial measures provide useful and relevant information regarding our performance and improve our ability to assess our financial condition. While similar measures are widely used in the industry in which we operate, the financial measures we use may not be comparable to other similarly titled measures used by other companies, nor are they intended to be substitutes for measures of financial performance or financial position as prepared in accordance with IFRS.
Constant Currency Revenue
Constant Currency Revenue is defined as revenue excluding the impact of fluctuations in currency exchange rates occurring when the financial results of foreign subsidiaries are converted into the Group's functional currency (i.e., Euro). Constant Currency Revenue is presented to aid management in their analysis of the performance of the Group and to assist in the comparison of our performance with the prior periods. We believe providing constant currency information provides valuable supplemental information regarding our revenue. We calculate constant currency revenue by converting our current period local currency revenue using the prior period foreign currency average exchange rates and comparing these adjusted amounts to our prior period reported revenue. The following tables set forth the calculation of Constant Currency Revenue for the fiscal year ended December 31, 2023 and provide a reconciliation to the most comparable IFRS measure, Revenue.
85
|
|
(Amounts in € millions, except as indicated otherwise) |
|
|||||||||||||||||
|
|
Biopharmaceutical and Diagnostic Solutions |
|
|
Engineering |
|
|
Consolidated |
|
|||||||||||
For the year ended December 31, 2023 |
|
High-Value Solutions |
|
|
Other containment and delivery solutions |
|
|
Total Biopharmaceutical and Diagnostic Solutions |
|
|
Total Engineering |
|
|
Total Consolidated |
|
|||||
Reported Revenue |
|
|
366.4 |
|
|
|
512.9 |
|
|
|
879.3 |
|
|
|
206.1 |
|
|
|
1,085.4 |
|
Effect of changes in currency translation rates |
|
|
4.4 |
|
|
|
3.8 |
|
|
|
8.2 |
|
|
|
0.2 |
|
|
|
8.4 |
|
Constant Currency Revenue |
|
|
370.8 |
|
|
|
516.7 |
|
|
|
887.5 |
|
|
|
206.3 |
|
|
|
1,093.8 |
|
|
|
(Amounts in € millions, except as indicated otherwise) |
|
|||||||||||||||||
|
|
Biopharmaceutical and Diagnostic Solutions |
|
|
Engineering |
|
|
Consolidated |
|
|||||||||||
Change in revenue at constant currency |
|
High-Value Solutions |
|
|
Other containment and delivery solutions |
|
|
Total Biopharmaceutical and Diagnostic Solutions |
|
|
Total Engineering |
|
|
Total Consolidated |
|
|||||
Constant Currency Revenue for the year ended December 31, 2023 |
|
|
370.8 |
|
|
|
516.7 |
|
|
|
887.5 |
|
|
|
206.3 |
|
|
|
1,093.8 |
|
Reported Revenue for the year ended December 31, 2022 |
|
|
293.2 |
|
|
|
506.4 |
|
|
|
799.7 |
|
|
|
184.0 |
|
|
|
983.7 |
|
Change in revenue at constant currency |
|
|
77.6 |
|
|
|
10.3 |
|
|
|
87.8 |
|
|
|
22.3 |
|
|
|
110.1 |
|
% Change in revenue at constant currency |
|
|
26.5 |
% |
|
|
2.0 |
% |
|
|
11.0 |
% |
|
|
12.1 |
% |
|
|
11.2 |
% |
EBITDA, Adjusted EBITDA and Adjusted EBITDA Margin
EBITDA is defined as net profit before income taxes, finance income, finance expense, depreciation and amortization. Adjusted EBITDA is defined as EBITDA as adjusted for certain income and costs that are unrelated to the underlying performance of the business, and that management considers not reflective of ongoing operational activities of the Company. EBITDA is presented to aid management in their analysis of the performance of the Group and to assist in the comparison of our performance with that of our competitors. Adjusted EBITDA is provided in order to present how the underlying business has performed excluding the impact of certain significant items that management considers not reflective of underlying operating activities and which may alter the underlying performance and impair comparability of results between periods.
86
The following table sets forth the calculation of EBITDA, Adjusted EBITDA, Adjusted EBITDA Margin for the fiscal years ended December 31, 2023 and 2022 and provides a reconciliation of these non-GAAP measures to the most comparable IFRS measures, Net Profit and Net Profit Margin. Adjusted EBITDA margin is calculated by dividing Adjusted EBITDA for a period by total revenue for the same period.
|
|
(Amounts in € millions, except as indicated otherwise) |
||||
|
|
For the year |
|
Change |
||
|
|
2023 |
|
2022 |
|
% |
Net Profit |
|
145.7 |
|
143.0 |
|
1.9% |
Income Taxes |
|
43.9 |
|
44.6 |
|
(1.7)% |
Finance Income |
|
(20.3) |
|
(25.0) |
|
(19.2)% |
Finance Expenses |
|
31.4 |
|
29.8 |
|
5.3% |
Operating Profit |
|
200.7 |
|
192.4 |
|
4.3% |
Depreciation and Amortization |
|
78.5 |
|
64.8 |
|
21.1% |
EBITDA |
|
279.2 |
|
257.3 |
|
8.5% |
Adjusting items |
|
12.3 |
|
6.3 |
|
95.2% |
Adjusted EBITDA |
|
291.5 |
|
263.6 |
|
10.6% |
|
|
|
|
|
|
|
Revenue |
|
1,085.4 |
|
983.7 |
|
|
Net Profit Margin (Net Profit/ Revenue) |
|
13.4% |
|
14.5% |
|
|
Adjusted EBITDA Margin (Adjusted EBITDA/ Revenue) |
|
26.9% |
|
26.8% |
|
|
87
Adjusted Operating Profit, Adjusted Operating Profit Margin, Adjusted Net Profit and Adjusted Diluted EPS
Adjusted Operating Profit, Adjusted Income Taxes, Adjusted Net Profit and Adjusted Diluted EPS represent respectively Operating Profit, Income Taxes, Net Profit and Diluted EPS as adjusted for certain income and costs expected to occur infrequently, and that management considers not reflective of ongoing operational activities. Adjusted Operating Profit, Adjusted Income Taxes, Adjusted Net Profit and Adjusted Diluted EPS are provided in order to present how the underlying business has performed excluding the impact of the adjusting items, which may alter the underlying performance and impair comparability of results between the periods.
The following table sets forth the reconciliation of EBITDA, Operating Profit, Income Taxes, Net Profit, Diluted EPS with Adjusted EBITDA, Adjusted Operating Profit, Adjusted Income Taxes, Adjusted Net Profit and Adjusted Diluted EPS for the fiscal years ended December 31, 2023 and 2022.
For further information on non-recurring items see “Cost of Sales” and “General and administrative expense” above.
|
|
|
||||||||
|
|
(Amounts in € millions, except as indicated otherwise) |
||||||||
For the year ended December 31, 2023 |
|
EBITDA |
|
Operating Profit |
|
Income Taxes (3) |
|
Net Profit |
|
Diluted EPS |
Reported |
|
279.2 |
|
200.7 |
|
43.9 |
|
145.7 |
|
0.55 |
Adjusting items: |
|
|
|
|
|
|
|
|
|
|
Start-up costs new plants (1) |
|
12.0 |
|
12.0 |
|
3.2 |
|
8.8 |
|
0.03 |
Restructuring and related charges (2) |
|
0.3 |
|
0.3 |
|
0.1 |
|
0.2 |
|
0.00 |
Adjusted |
|
291.5 |
|
213.0 |
|
47.2 |
|
154.7 |
|
0.58 |
|
|
|
||||||||
|
|
(Amounts in € millions, except as indicated otherwise) |
||||||||
For the year ended December 31, 2022 |
|
EBITDA |
|
Operating Profit |
|
Income Taxes (3) |
|
Net Profit |
|
Diluted EPS |
Reported |
|
257.3 |
|
192.4 |
|
44.6 |
|
143.0 |
|
0.54 |
Adjusting items: |
|
|
|
|
|
|
|
|
|
|
Start-up costs U.S. plant (1) |
|
6.2 |
|
6.2 |
|
1.6 |
|
4.6 |
|
0.02 |
Restructuring and related charges (2) |
|
0.1 |
|
0.1 |
|
— |
|
0.1 |
|
0.00 |
Adjusted |
|
263.6 |
|
198.7 |
|
46.2 |
|
147.7 |
|
0.56 |
The following table sets forth the calculation of Adjusted Operating Profit Margin and provides a reconciliation of these non-GAAP measures to the most comparable IFRS measure, Operating Profit Margin. Adjusted Operating Profit margin is calculated by dividing Adjusted Operating Profit for a period by total revenue for the same period.
88
|
|
(Amounts in € millions, except as indicated otherwise) |
|
|||||
|
|
For the year |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Revenue |
|
|
1,085.4 |
|
|
|
983.7 |
|
Operating Profit Margin (Operating Profit/ Revenue) |
|
|
18.5 |
% |
|
|
19.6 |
% |
Adjusted Operating Profit Margin (Adjusted Operating Profit/ Revenue) |
|
|
19.6 |
% |
|
|
20.2 |
% |
CAPEX
Capital Expenditure, or CAPEX, is the sum of investment amounts in property, plant and equipment and intangible assets during the period (excluding right-of-use assets recognized during the period in accordance with IFRS 16 Leases). These investment activities consist of acquisitions of property, plant and equipment and intangible assets, excluding the grants which may take the form of a transfer of a non-monetary asset (such as land).
The following table sets forth the CAPEX for the fiscal years ended December 31, 2023 and 2022:
|
|
(Amounts in € millions, except as indicated otherwise) |
||||
|
|
For the year |
|
Change |
||
|
|
2023 |
|
2022 |
|
€ |
Addition to Property, plants and equipment (1) |
|
444.6 |
|
294.5 |
|
150.1 |
Addition to Intangible Assets |
|
8.7 |
|
8.1 |
|
0.6 |
CAPEX |
|
453.3 |
|
302.6 |
|
150.7 |
See Note 17 “Intangible Assets” and Note 18 “Property, plant and equipment” to the Consolidated Financial Statements for additional details.
For further information on Capital Expenditure see “Liquidity and Capital Resources - Capital Expenditure” above.
Free Cash Flow
Free Cash Flow is defined as cash flows from operating activities excluding interests paid and received, less investments in property, plant and equipment and intangible assets on a paid-out cash basis.
The following table sets forth the calculation of Free Cash Flow for the fiscal years ended December 31, 2023 and 2022:
|
|
(Amounts in € millions, except as indicated otherwise) |
||||
|
|
For the year |
|
Change |
||
|
|
2023 |
|
2022 |
|
€ |
Cash Flow from Operating Activities |
|
105.2 |
|
103.3 |
|
1.9 |
Interest paid |
|
3.1 |
|
3.5 |
|
(0.4) |
Interest received |
|
(0.9) |
|
(0.8) |
|
(0.1) |
Purchase of property, plant and equipment |
|
(433.2) |
|
(235.0) |
|
(198.2) |
Proceeds from sale of property plant and equipment |
|
0.6 |
|
0.1 |
|
0.5 |
Purchase of intangible assets |
|
(8.7) |
|
(8.1) |
|
(0.6) |
Free Cash Flow |
|
(333.9) |
|
(137.0) |
|
(196.9) |
89
For further information on cash flow see “Liquidity and Capital Resources - Cash Flow” above.
Net (Debt)/ Cash
The following table sets forth the calculation of Net (Debt)/ Cash, which is a metric used by the management to analyze the financial stability of our business. Net (Debt)/ Cash is calculated as the sum of our current and non-current financial liabilities and less the sum of the other current financial assets, other non-current financial assets - Fair value of derivatives financial instruments and cash and cash equivalents.
|
|
(Amounts in € millions, except as indicated otherwise) |
||
|
|
At December 31, |
|
At December 31, |
|
|
2023 |
|
2022 |
Non-current financial liabilities |
|
(255.6) |
|
(148.4) |
Current financial liabilities |
|
(143.3) |
|
(70.7) |
Other non-current financial assets - Fair value of derivatives financial instruments |
|
0.6 |
|
2.8 |
Other current financial assets |
|
4.4 |
|
33.6 |
Cash and cash equivalents |
|
69.6 |
|
228.7 |
Net (Debt)/ Cash |
|
(324.4) |
|
46.0 |
90
Capital Employed
The following table sets forth the reclassified consolidated statements of financial position which is presented to aid management in their analysis of the Capital Employed to generate profits. Capital Employed is determined as the sum of non-current assets (excluding the fair value of derivatives financial instruments) and net working capital, less the sum of provisions and non-current liabilities (excluding non-current advances from customers). Net working capital is the difference between current assets and current liabilities, excluding current financial assets, current financial liabilities and cash and cash equivalents, to which the non-current advances from customers are added.
|
|
|
|
|
|
|
(Amounts in € millions, except as indicated otherwise) |
||
|
|
At December 31, |
|
At December 31, |
|
|
2023 |
|
2022 |
- Goodwill and Other intangible assets |
|
81.0 |
|
79.4 |
- Right of use assets |
|
18.2 |
|
19.3 |
- Property, plant and equipment |
|
1,028.5 |
|
641.4 |
- Financial assets - investments FVTPL |
|
0.7 |
|
0.8 |
- Other non-current financial assets |
|
4.5 |
|
1.0 |
- Deferred tax assets |
|
76.3 |
|
69.2 |
Non-current assets excluding FV of derivative financial instruments |
|
1,209.2 |
|
811.1 |
|
|
|
|
|
- Inventories |
|
255.3 |
|
213.3 |
- Contract assets |
|
172.6 |
|
103.4 |
- Trade receivables |
|
301.8 |
|
212.7 |
- Trade payables |
|
(277.8) |
|
(239.2) |
- Advances from customers |
|
(22.9) |
|
(26.6) |
- Non-current advances from customers |
|
(39.4) |
|
— |
- Contract liabilities |
|
(22.3) |
|
(14.8) |
Trade working capital |
|
367.2 |
|
248.8 |
|
|
|
|
|
- Tax receivables and Other receivables |
|
58.2 |
|
54.0 |
- Tax payables and Other liabilities |
|
(107.0) |
|
(111.2) |
- Current provisions |
|
(1.1) |
|
— |
Net working capital |
|
317.4 |
|
191.7 |
|
|
|
|
|
- Deferred tax liabilities |
|
(9.6) |
|
(21.0) |
- Employees benefits |
|
(7.4) |
|
(8.3) |
- Non-current provisions |
|
(4.0) |
|
(5.5) |
- Other non-current liabilities |
|
(48.5) |
|
(18.1) |
Total non-current liabilities and provisions |
|
(69.5) |
|
(52.9) |
|
|
|
|
|
Capital employed |
|
1,457.1 |
|
949.9 |
|
|
|
|
|
Net (Debt)/ Cash |
|
(324.4) |
|
46.0 |
|
|
|
|
|
Equity |
|
(1,132.6) |
|
(995.9) |
|
|
|
|
|
Total Equity and Net (Debt)/ Cash |
|
(1,457.1) |
|
(949.9) |
|
|
|
|
|
91
Backlog
Our backlog represents, as of a point in time, estimated future revenue for work not yet completed under (i) specific purchase orders, with regards to our Biopharmaceutical and Diagnostic Solutions segment; and (ii) certain one-off agreements, with regards to our Engineering segment. We recognize direct revenue over the life of the contract based on our performance of services under the contract. Contracts may be terminated or delayed by our customers or regulatory authorities for reasons beyond our control. To the extent projects are delayed, the timing of our revenue could be affected. In the event a customer terminates a contract, we are generally entitled to be paid for services rendered through the termination date and for services provided in winding down the project. However, we are generally not entitled to receive the full amount of direct revenue reflected in our backlog in the event of a contract termination. The duration of the projects in our backlog, and the related revenue recognition, ranges from several months to a couple of years. For orders that are placed inside a contractual firm period, we generally have a contractual right to payment in the event of cancellation. Fluctuations in our reported backlog levels also result from the timing and order pattern of our customers who often seek to manage their level of inventory on hand.
Because of customer ordering patterns, our backlog reported for certain periods may fluctuate and may not be indicative of future results. A number of factors may affect backlog and the direct revenue generated from our backlog, including: (a) the size, complexity and duration of projects; and (b) the cancellation or delay of projects.
Our backlog as of December 31, 2023 was approximately €944.9 million, compared to a total backlog of €957.0 million as of December 31, 2022. During the year ended December 31, 2023, we had a new order intake of approximately €1,073.2 million compared to approximately €1,060.7 million for the year ended December 31, 2022. The Group currently anticipates that there may be fluctuations quarter to quarter in order intake and backlog.
Although an increase in backlog will generally result in an increase in future direct revenue to be recognized over time (depending on future contract modifications, contract cancellations and other adjustments), an increase in backlog at a particular point in time does not necessarily correspond to an increase in direct revenues during a particular period. The timing and extent to which backlog will result in direct revenue depends on many factors, including the timing of commencement of work, the rate at which we perform services, scope changes, cancellations, delays, receipt of regulatory approvals and the nature, duration, size, complexity and phase of the studies. In addition, delayed projects remain in backlog until they are canceled. As a result of these factors, our backlog is not necessarily a reliable indicator of future direct revenue and we might not realize all or any part of the direct revenue from the authorizations in backlog as of any point in time.
C. RESEARCH AND DEVELOPMENT, PATENTS AND LICENSES, ETC.
See “Item 4. Information on the Company—B. Business Overview.”
D. TREND INFORMATION
Other than as disclosed elsewhere in this annual report, we are not aware of any trends, uncertainties, demands, commitments or events for the 2023 fiscal year that are reasonably likely to have a material adverse effect on our revenue, income, profitability, liquidity or capital resources, or that caused the disclosed financial information to be not necessarily indicative of future operating results or financial condition.
E. CRITICAL ACCOUNTING ESTIMATES
See "Significant judgments and estimates" in our Consolidated Financial Statements.
92
ITEM 6. Directors, Senior Management and Employees
The following table sets forth information regarding the directors and executive officers of the Company.
Directors and Executive Officers |
Age |
Position/Title |
Sergio Stevanato |
80 |
Director—Emeritus Chairman |
Franco Stevanato |
50 |
Director—Executive Chairman |
Marco Stevanato |
51 |
Director—Vice-Chairman |
Fabiano Nicoletti |
80 |
Director |
Alvise Spinazzi |
50 |
Director |
Fabrizio Bonanni |
77 |
Director |
Fabio Buttignon |
64 |
Director |
Madhavan Balachandran |
72 |
Director |
Donald Eugene Morel Jr. |
66 |
Director |
William Federici |
64 |
Director |
Paola Vezzaro |
55 |
Director |
Franco Moro |
61 |
Director and Chief Executive Officer |
Marco Dal Lago |
51 |
Chief Financial Officer |
Mauro Stocchi |
57 |
Chief Business Officer |
Paolo Patri(1) |
54 |
Chief Technology Officer |
Riccardo Butta |
58 |
President Americas |
(1) Effective February 29, 2024, Mr. Patri departed Stevanato Group to pursue other opportunities.
Sergio Stevanato. Son of Giovanni Stevanato, founder of the Stevanato Group, Sergio Stevanato has been actively involved in the family business since high-school. He graduated in law from the University of Ferrara in 1969, to then take the leadership of the company. He has spent his whole career in the family business of which he is currently the Emeritus Chairman. In 2007 he was awarded by the President of the Italian Republic the honorary recognition of Knight of Labor (Cavaliere del Lavoro) for his achievements as an entrepreneur.
Franco Stevanato. Son of Sergio Stevanato, Franco Stevanato graduated in Political Science from the University of Trieste in 1998 and attended an Advanced Management Program at the Kellogg School of Management in 2015. During his university years, he gained professional experience in the sales department of Saint Gobain in France. Upon completing his studies, he joined the family business, initially taking up a role in sales. Over the years, he has been the key figure and driving force behind the internationalization of the Company and its continuing development from product diversification – via strategic acquisitions and in-house innovations – to enhanced managerial processes and structural improvements. He also contributed to improving the Stevanato Group’s corporate governance by building an effective infrastructure to support decision making and promoting a skills-based board that benefits from specialist expertise and meaningful perspective. He was CEO of the Group from 2010 to 2020. Since 2021, he has been the Executive Chairman of the Board.
93
Marco Stevanato. Son of Sergio Stevanato, Marco Stevanato graduated in Business Administration from the University of Trieste in 1998. After graduation, he gained experience in Germany, Belgium and the United States in the Finance & Controlling department of a German multinational company to then join the family business in 1999. In 2006 he was appointed Vice President of the Stevanato Group and has led the internationalization projects and development of the plants in Monterrey (Mexico), Zhangjiagang (China) and Sete Lagoas (Brazil). He also serves as Chief Executive Officer of SFEM ITALIA S.r.l., the Family Office that manages the investments of the Stevanato family, not related to the industrial group.
Fabiano Nicoletti. Born in Venice in 1943, Fabiano Nicoletti graduated in Solid State Physics from the University of Padua in 1972. He gained more than forty years of experience at Stazione Sperimentale del Vetro (the Italian State Glass Research Institute) in Venice. For a long time, he collaborated in several international committees and working groups and he was one of the founders (and President) of the European Society of Glass Science and Technology (ESG), the President of USTV (Union Scientifique et Technologique du Verre), as well as the President of the ICG (International Commission on Glass) of which he is Honorary President. Both in 1983 and 1993, he was awarded by the President of the Italian Republic the honorary recognitions of “Cavaliere Ordine al Merito della Repubblica” and of “Ufficiale Ordine al Merito della Repubblica”, respectively. He has been a member of the board of directors of Stevanato Group since 2003.
Alvise Spinazzi. Graduated in Law from the University of Padua in 1997, he obtained an LL.M. in International Business and Trade Law from Fordham University School of Law in New York. He qualified as a lawyer in New York in 2000 and in Italy in 2001. Before founding with other partners the law firm SAT Studio Legale in Padua in 2007, he practiced in the New York office of the international law firm Simpson Thacher & Bartlett and in the Milan office of Italian law firm Chiomenti. He has been a member of the board of directors of Stevanato Group since 2011.
Fabrizio Bonanni. Holding a doctorate in chemistry, summa cum laude, mention of honor, from the University of Florence, Italy, Mr. Bonanni carried out postdoctoral work in physiological chemistry at the Massachusetts Institute of Technology. He is an alumnus of the Institute for International Management, Northwestern University, J.L. Kellogg Graduate School of Management and of the Executive Program in Manufacturing, Harvard University, Graduate School of Business Administration. He spent 25 years at Baxter International in Italy, Belgium and the U.S. reaching the positions of corporate vice president Quality System and CVP Regulatory and Clinical Affairs. From 1999 to 2013, he served in senior executive roles at Amgen, including senior vice president, Quality and Compliance and corporate compliance officer, senior vice president, Manufacturing and executive vice president, Operations. Currently, he is a member of the board of Incog BioPharma Services, a director of UCLA’s Technology Development Corporation and serves on the Advisory Board of InCube Labs of San Jose, California. His past board memberships include XBiotech, where he chaired the Audit Committee, Menarini Biotech, and Theranos, where he chaired the Compliance and Quality Committee. He has been a member of the board of directors of Stevanato Group since 2013.
Fabio Buttignon. Graduated in Economics and Business Administration from the University Ca’ Foscari of Venice in 1983. He carried out research activities in Finance and Strategy at the University of California, Los Angeles. He was research fellow, assistant professor and associate professor of Business Administration at the University Ca’ Foscari of Venice. Mr. Buttignon is currently full professor of Corporate Finance at the University of Padua, Department of Economics and Management. Qualified as Dottore Commercialista and Revisore dei Conti (CPA and Statutory Auditor), he is founder and managing partner of Buttignon Zotti Milan & Co., a financial advisory boutique specialized in corporate finance and accounting services. He has been a member of the board of directors of Stevanato Group since 2014.
Madhavan Balachandran. Holding a Master of Science degree in Chemical Engineering from The State University of New York at Buffalo and an MBA from East Carolina University, Mr. Balachandran is Chief Operating Officer of Nutcracker Therapeutics, a developer of mRNA therapeutics, a position he has held since September 2020. He previously served as Chief Executive Officer of ADRx, Inc., a pre-clinical stage biotechnology company, since August 2019. Prior to that, he was Executive Vice President, Operations of Amgen Inc. from August 2012 until July 2016 and retired as an Executive Vice President in January 2017, having served in various management positions since joining the company in 1997. Prior to his tenure at Amgen, Mr. Balachandran held leadership positions at Copley Pharmaceuticals, now a part of Teva Pharmaceuticals Industries Ltd. and Burroughs Welcome Company, a predecessor before mergers of GlaxoSmithKline plc. He currently serves as a director in Catalent Inc., uniQure NV and A2 Biotherapeutics, Inc. He has been a member of the board of directors of Stevanato Group since 2018.
94
Donald Eugene Morel Jr. Holding BS degree in Metallurgical Engineering from Lafayette, an MS in Materials Science and a Ph.D. in Materials Science and Veterinary Medicine from Cornell University, Dr. Morel also completed the Executive Program at Darden School of Business—University of Virginia. After gaining experience in a broad range of space related research programs focused on advanced satellite systems, Dr. Morel joined West Pharmaceutical Services, Inc., where he served as Chairman from April 2003 and Chief Executive Officer from April 2002 until his retirement in June of 2015. Dr. Morel has authored or co-authored over thirty scientific publications and was elected a fellow of the American Institute for Medical & Biologic Engineering. he currently serves as a member of the board of directors in Catalent Inc. and Integra Life Sciences Holdings. He has been a member of the board of directors of Stevanato Group since 2018.
William Federici. Holding a BA in Economics from Rutgers University, Livingston College and an MBA in Professional Accounting from Rutgers University, he is a member of the American Institute of Certified Public Accountants. Mr. Federici was a member of the board of directors of Zynerba Pharmaceuticals, Inc., a Specialty Pharmaceutical, U.S. public company, where he served as Audit Committee Board Chair from 2015 until it was sold to Harmony Biosciences Holdings, Inc. in October of 2023. Mr. Federici joined West Pharmaceutical Services, Inc., a NYSE traded U.S. public company, in 2003 as Chief Financial Officer after more than 20 years’ experience in public accounting primarily serving the Pharmaceutical Industry. He retired from West Pharmaceutical Services, Inc. in 2018. He has been as member of the board of directors of Stevanato Group since May 2021.
Paola Vezzaro. Graduated, summa cum laude, in Business Administration from the University Commerciale “Luigi Bocconi” in Milan in 1993 and, subsequently, graduated in Political Science and Sociology from the University of Milan. She also obtained a Master in Human Resources from Cattolica University in Milan. Mrs. Vezzaro has an extensive experience in the HR fields, having served as HR director in many primary companies. She joined Engie in 2011, where she has held several HR high-profile international positions. Since July 2019, she has been serving as Chief Human Resources and Health & Safety Officer North, South and Eastern Europe for Engie. She has been a member of the board of directors of Stevanato Group since May 2021.
Franco Moro. Graduated in Chemical Engineering from the University of Padua in 1987, he obtained an MBA from SDA Bocconi in Milan. Mr. Moro has gained significant experience managing global manufacturing companies for over 30 years. He has worked as plant director of FIS—Fabbrica Italiana Sintetici and then of Cambrex Profarmaco Milano, before taking over as Chief Executive Officer of FIS—Fabbrica Italiana Sintetici from 2010 to 2018. Mr. Moro joined Stevanato Group in 2019 and after serving as Chief Operating Officer for 2 years, was appointed as Chief Executive Officer in February 2021. He has been a member of the board of directors of Stevanato Group since February 2021.
Marco Dal Lago. Graduated from Ca’ Foscari University of Venice in 1997 with a degree in Business Administration. Mr. Dal Lago joined Stevanato Group in January 2020, after about 25 years of experience in the fields of controlling, finance, administration, compliance and risk management, working in multinational industrial companies and coordinating multi-year planning and mergers & acquisitions processes. Mr. Dal Lago is currently Chief Financial Officer at Stevanato Group, with responsibility for the organization, supervision and guarantee of Group administration, finance and controlling activities.
Mauro Stocchi. Graduated from Ca’ Foscari University of Venice in 1991 and holds a Masters of Business Administration from SDA Bocconi in Milan. Mr. Stocchi commenced his career in De Longhi S.p.A. followed by a 10-year period within the Siemens Group. He joined Stevanato Group in 2004 and in 2008, Mr. Stocchi was appointed CFO of the Group while retaining responsibility over business development activities. From 2010, he covered the position of Corporate General Manager with direct responsibility for all corporate functions. He also served as General Manager of the Pharmaceutical System Division and is currently Chief Business Officer of the Group with responsibilities of strategic business development, sales, product management, marketing and communication and drug delivery systems business.
Paolo Patri. Graduated from the University of Milan in 1995 with a degree in Chemistry. Mr. Patri has over 20 years of experience in the pharmaceutical industry, both in production and in the development of pharmaceuticals and biotech, gaining a significant track record of achieving global regulatory approvals for both large, small molecules and combination medicinal product through standard and accelerated programs. Mr. Patri held various positions at different international organizations, including Cambrex Profarmaco, Janseen-Cilag a Johnson & Johnson company, Chiesi Farmaceutici and Dompé Farmaceutici. In Chiesi Farmaceutici he held from 2008 to 2017 the role of Global
95
Head of CMC (Chemistry, Manufacturing and Controls). In Dompé Farmaceutici, he held the role of Chief Manufacturing Officer. Mr. Patri joined Stevanato Group in October 2018 and has since assumed the role of Chief Technology Officer, overseeing the management of the research and development department, as well as investments, projects and other activities supporting the Group vision. Mr. Patri is also currently Chief Executive Officer of Medirio SA, a wholly-owned indirect Swiss subsidiary of Stevanato Group. Effective February 29, 2024, Mr. Patri departed Stevanato Group to pursue other opportunities.
Riccardo Butta. Riccardo Butta holds a master’s degree in mechanical engineering from Politecnico University of Milan, a degree in business management and innovation from MIP in Milan and successfully completed the Executive Leadership Development Program from Stanford University in Palo Alto, California. Riccardo was the Senior Vice President of Flex Health Solutions, responsible for the global commercial organization of a Flex business unit providing contract design, manufacturing, and logistics services to the healthcare industry with focus on medical devices, drug delivery solutions, diagnostics and life sciences equipment. During his tenure at Flex, Riccardo was also responsible for the European organization of Flex Health Solutions, for the global device development organization, and for scale-up and management of the design center in Milan. In February 2022, Riccardo joined Stevanato Group as President Americas, overseeing the growth of the Group in the Americas, as well as the expansion of the commercial offering around high value products and services.
Compensation Report
As a matter of Italian law, the compensation of executive directors is determined by the Board of Directors, while the Company's shareholders generally determine the base compensation for all Board members, including non-executive directors.
The aggregate compensation for members of our board of directors (including pension expense and long- term benefits) was €2,762 thousand for the year ended December 31, 2023.
The aggregate compensation for members of our key management personnel (excluding Chairman and including Chief Executive Officer) was €2,838 thousand for the year ended December 31, 2023. The compensation for each of our key management personnel consists of the following elements: base salary, fringe benefit, bonus based on revenue and KPI-based bonus, pension expense and share-based payments.
Stock Grant Plan
On March 4, 2021, we approved by means of resolution of the ordinary shareholders’ meeting, a restricted stock grant plan (the “Stock Grant Plan”) with a duration of six years, running from January 1, 2021 until December 31, 2026, which is governed by its own regulation (the “Regulation”). The total amount of ordinary shares available for granting under the Stock Grant Plan will constitute 0.5% of the issued share capital as of January 1, 2021.
The Stock Grant Plan provides for (i) the right of the beneficiaries to be granted a certain number of ordinary shares of the Company, free of any charges; and (ii) the right of the beneficiaries to be granted a further number of ordinary shares of the Company, in the event that certain over-performances with respect to the Company financial targets have been met.
Both the shares granted as described sub (i) above and those granted as described sub (ii) above, are subject to the lock-up period and the call option right of the Company, described below.
Eligibility, Awards, and Administration
Those eligible to participate in the Stock Grant Plan are (i) any employees of either the Company, or any of its subsidiaries, and/or (ii) any self-employed individuals who work for either the Company, or any of its subsidiaries, who have been identified from time to time by the board of directors of the Company as holding a strategic role in either the company, or any of its subsidiaries.
The granting of the ordinary shares to the beneficiaries is subject to each of them meeting, by the date of the offer of shares, the following requirements:
96
Our board of directors is entrusted with managing the Stock Grant Plan. In particular, in compliance with the Regulation, our board of directors has the power, inter alia, to identify the beneficiaries of the Stock Grant Plan among those employees or self-employed individuals who have taken on a strategic role within the Company’s group, and to determine the number of ordinary shares to be granted to the beneficiaries.
Vesting and Holding
The Stock Grant Plan provides for three two-year vesting periods: one from January 1, 2021 to December 31, 2022, one from January 1, 2023 to December 31, 2024, and one from January 1, 2025 to December 31, 2026. At the beginning of each of the vesting periods, the Company will grant, free of any charges, to the beneficiaries (except for the tax charges financed by the Company), a certain amount of its ordinary shares, which shall be indicated in the relevant grant letters addressed to the beneficiaries.
Lock-up Period
The Stock Grant Plan provides for three one-year lock-up periods, starting at the end of each of the three respective vesting periods.
Until the expiry of the relevant lock-up periods, the beneficiaries, or any of their heirs in case of the beneficiaries’ death, may not transfer the ordinary shares they have been granted in the prior vesting period to individuals and/or entities other than the Company. Furthermore, until the end of each lock-up period, each beneficiary must keep the shares free from any options or pre-emption rights or any other restriction or limitation, contractual or otherwise, except for those restrictions or limitations arising from the Company’s articles of association and/or the Stock Grant Plan.
Call Option
Pursuant to the Regulation, each of the beneficiaries enters into a separate call option agreement with the Company, by means of which each of them undertakes, irrevocably, to sell to the Company, all or part of the ordinary shares they have been granted, in the event that the Company decides to exercise its call option right.
The Company is entitled to exercise its call option right subject to the occurrence of at least one of the following events:
With respect to the events sub (a) above, the Company shall be entitled to exercise its call option right on all the ordinary shares with which the concerned beneficiary has been granted; while, with respect to the events sub (b), the Company shall be entitled to exercise its call option right on a percentage of the ordinary shares with which the concerned beneficiary has been granted, which depends on the extent to which the cumulative financial turnover has departed from the Company’s financial targets.
97
Claw-back
Within two years from the end of the lock-up period, the Company shall be entitled to request from the beneficiaries the restitution, in whole or in part, of either the granted ordinary shares and/or the further amount of granted ordinary shares, in the event that the degree of achievement of the targets set forth in the business plan of the Company related to a vesting period, has been calculated on the basis of data that subsequently turned out to be erroneous and the differences between this data used and the adjusted data are such as to have caused the non-exercise of the call option right by the company. In the event that the ordinary shares have already been sold by the beneficiaries to third-parties, the Company will have the right to request the restitution of the sale value of the ordinary shares to such beneficiaries.
Certain Transactions
Our board of directors and/or our shareholders’ meeting, should it be required or appropriate in connection with extraordinary transactions, events or special circumstances concerning the Company, or any of its subsidiaries, has the right to revoke the Stock Grant Plan or suspend its execution by means of a thirty-day prior written notice to be sent to the Stock Grant Plan’s beneficiaries.
In case of revocation or suspension of the Stock Grant Plan, the Company shall grant each of the beneficiaries with a different kind of incentive, unless, after the conclusion of the concerned extraordinary transaction, event or special circumstance, our board of directors and/or our shareholders’ meeting will issue a new incentive plan in the event of revocation of the Stock Grant Plan, or the reactivation of the latter in the event of its suspension, in both cases in such a way as to ensure that the granted incentives shall be substantially equivalent to those that would have been due to each beneficiary pursuant to the Stock Grant Plan.
Amendment to Stock Grant Plan 2021-2027
On April 11, 2022 the Board of Directors approved an amendment to the "Restricted Stock Grant Plan Stevanato Group S.p.A. 2021-2027", in order to allow the entry of new beneficiaries in 2022, previously involved in the compensation plan called "Long Term Incentive 2020-2023". In accordance with specific rules, existing and new beneficiaries relating to the first vesting period coexist during 2022 under the "Restricted Stock Grant Plan Stevanato Group S.p.A. 2021-2027". Through such amendment, beneficiaries of the Long Term Incentive 2020-2023 were offered the possibility of becoming beneficiaries of the "Restricted Stock Grant Plan Stevanato Group S.p.A. 2021-2027" in 2022 by using the rights deriving from the participation in the Long-Term Incentive 2020-2023 in the meantime accrued to them, but providing, that the free of charge transfer of the property of a certain number of Stevanato Group S.p.A. shares should have been done after the end of the First Vesting Period, after having verified the actual achievement of the Performance Objectives (in terms of consolidated revenue and EBITDA) set for such vesting period and the continuation of the employment relationship.
On May 12, 2022, Stevanato Group S.p.A. sent, to the new beneficiaries of shares, a letter granting them the right to obtain the transfer free of charge of a certain number of shares if the targets, in terms of consolidated revenue and EBITDA provided for the vesting period in relation to which the shares were assigned, are achieved. New beneficiaries are individuals who play a strategic role in the Group, including its economic and strategic development, and the above right to transfer shares (subject to certain conditions) aligns their interests to those of the shareholders and other stakeholders of the Company, during the period between January 1, 2021 and December 31, 2026.
The effectiveness of the rights attributed to each of the new beneficiaries of shares is conditional upon the verification by the Stevanato Group's administrative body of the degree of achievement of the performance target provided in relation to the first vesting period after the end of the first vesting period. On the basis of this assessment the number of shares indicated in the letter of attribution of rights can be reduced based on the degree of target achievement.
In May 2023 the Company formally communicated to the initial beneficiaries and to the new beneficiaries the achievement of the performance objectives, assigning accordingly the shares additionally assigned and the shares, as reported respectively in the additional grant letter – to the initial beneficiaries – and in the grant letter, sent to the new beneficiaries.
98
Long Term Incentive Plan
On December 15, 2022, we approved by means of resolution taken by the Board of the Directors of the Company the Restricted Share Plan 2023-2027 (the “RSP”) and the Performance Share Plan 2023-2027 (the “PSP”) with a duration of 5 years, running from January 1, 2023 until December 31, 2027, which are governed by their own regulation (the “Long Term Plan Regulation”). The Plan has a vesting period of three-years: the first cycle from January 1, 2023, to December 31, 2025, the second cycle from January 1, 2024, to December 31, 2026, and the third cycle from January 1, 2025, to December 31, 2027. The total number of SG ordinary shares available for granting under the first and the second cycle of RPS and PSP represents respectively less than 0.5% and 0.1% of the issued Company’s share capital as of January 1, 2023. The Company’s Board of Directors is responsible for the management of the PSP and RSP and may make any changes to the Long Term Plan Regulation, which it sees fit or believes to be useful or necessary to better target the objective and focus of the PSP and RSP. The Board of Directors of the Company – based upon the proposal formulated by the Compensation Committee – defined the mix of the two plans for beneficiaries; in the first two cycles, the mix between the two plans was 50% shares granted 50% as PSP and 50% as RSP.
The Performance Share Plan
The PSP provides for (i) the right of the Participants to receive a certain number of ordinary shares of the Company free of any charges, provided that certain conditions – in terms of achievement of specific Performance Targets (Revenue Growth and ROIC) and the existence of employment relationship or any other contract or document governing the relationship between the individual Participant and Stevanato Group (hereinafter the “Relationship”) – are met at the end of each of the three-year Performance Periods; and (ii) the right of the Participants to receive a further number of ordinary shares of the Company, in case of overachievement of the said Performance Targets. Both Performance Targets are equally weighted and envisage in case of achievement at maximum performance level a payout of 200% of target shares granted and a payout of 50% of target shares granted in case of achievement at minimum performance level.
Eligibility, Awards, and Administration
Those eligible to participate in the PSP are any employees of either the Company, or any of its Subsidiaries who have been identified from time to time by the Board of Directors of the Company –, based upon the proposal formulated by the Compensation Committee for C-Level roles and by the Chief Executive Officer of the Company for the other Beneficiaries – as holding a strategic role in either the Company, or any of its subsidiaries (Director and above position) and who will receive, at the beginning of each Vesting Period, the Grant Letter containing the awarding of the Right to Receive Shares and the Target Number of Shares he/she is entitled to receive if the above mentioned conditions are met.
Claw-back provision
The Participant will be required to return to the Company the shares received if during the two-years period following the date in which he/she received the shares it is proven that that the granting of the shares took place on the basis:
99
The Restricted Share Plan
The RSP provides for the right of the Participants to receive a certain number of ordinary shares of the Company free of any charges, provided that certain conditions – in terms of existence of employment relationship or any other contract or document governing the relationship between the individual Participant and Stevanato Group – are met at the end of each of the three Installment-Vesting Schedule.
Eligibility, Awards, and Administration
Those eligible to participate in the RSP are any employees of either the Company, or any of its Subsidiaries, who have been identified from time to time by the Board of Directors of the Company - , based upon the proposal formulated by the Compensation Committee for C-Level roles and by the Chief Executive Officer of the Company for the other Beneficiaries -, as holding a strategic role in either the company, or any of its subsidiaries (Director and above position) and who will receive, at the beginning of each Vesting Period, the Grant Letter containing the awarding of the Right to Receive Shares and the Target Number of Shares he/she is entitled to receive if the above mentioned conditions are met.
In particular, the Award of the Shares to each Participant after verification of the relevant Presence Condition will follow the Vesting Schedule described hereafter:
Claw-back provision
The Participant will be required to return to the Company the shares received if during the two-years period following the date in which he/she received the shares it is proven that that during the Presence Period the Participant adopted a behavior contrary to law and / or Company regulations (violation of the organization, management and control model and code of ethics or internal procedures of the Company).
Rules common to the PSP and the RSP
Good/ Bad leavership
The provisions regarding the termination of the Relationship differ in case the relevant Participant is a Good Leaver or Bad Leaver: in essence, while the good leaver retains the right to receive all or at least a certain number of shares, the bad leaver loses the right to receive the shares
Good Leaver means any hypothesis in which there is the termination of the Relationship as a result of:
100
All cases not listed in the aforesaid Good Leaver list are to be understood as included in the Bad Leaver cases. The Board of Directors, in its sole discretion, can determine that the relevant Participant is not a Bad Leaver.
Extraordinary transactions
In case of extraordinary transactions (such as mergers/ spin-off, transactions involving the Company’s share capital, etc.), the Board of Directors may make all the amendments and additions to PSP/RSP and / or their Regulation considered necessary or appropriate to keep the substantial and economic contents of the PSP/RSP unchanged, within the limits allowed by the applicable legislation in force at the time, including for the PSP the revision of the performance conditions through, inter alia, the revision of the target levels or of the Performance Criteria. The PSP / RSP Regulation may be amended by the Board of Directors at any time, provided that, except for adjustments or amendments permitted or required by the PSP/RSP Regulation, no such amendment, without the written consent of the Participant, will materially adversely affect the rights of the Participant granted by the PSP/RSP Regulation.
Changes to the corporate structure
If during the Vesting Period a Change of Control should occur, the Board of Directors, at its sole discretion, will have the right to award the Participants in advance with the entire number of Shares granted with the Right to Receive Shares, or part of it, and to provide for the early termination of the PSP/RSP with or without consideration.
The Shares may be awarded independently of the actual achievement of the Performance Conditions, only for PSP, and of the Presence Condition for both the PSP and RSP.
Tax treatment of the shares
The tax and social security charges resulting from the assignment of the Right to Receive Shares/awarding of the Shares are borne by the Participants and by the Company in compliance with their respective obligations based on pro tempore existing legislation. Each Participant, throughout the life of the PSP/RSP, is personally responsible for obtaining information relating to the tax and social security treatment of the Shares that he or she is granted and should obtain and rely upon his or her own independent financial and tax advice.
Each Participant is liable for the payment of any social charges, income tax and any other taxes and/or charges he or she owes. He or she is entirely responsible for the consequences that could result from (i) the failure to file a declaration for which he or she is fully responsible, or (ii) filing an incomplete declaration with the tax authorities in the country in which he or she is a resident for tax purposes, or in any other country in which he or she may have tax obligations (especially in the case of mobility or resulting from the Participant’s citizenship).
If as a result of an Award of Shares or ensuing vesting or sale of Shares, the Company is required to act as withholding agent and pay or withhold taxes, social charges, or any other type of dues on behalf of the Participant, the Company will give to the Participant the possibility to pay his or her dues within a specific timeframe, either directly or through the sale of the required number of Shares simultaneously with or shortly following their release in order to use the proceeds to cover tax liabilities (“sell to cover”), to the extent that such sell to cover complies with applicable laws and Company regulations, including without limitation any insider trading laws or regulations; if he or she declines or fails to make such payment or fails to respond, the Company reserves the right to carry out a sale of a sufficient number of Shares to cover the payment of the sums owed with the proceeds derived from this sale to be paid directly to the Company.
Stock Ownership and Retention Guidelines for Senior Officers
These guidelines are designed to assist in focusing executives on the long-term success of Stevanato Group and on shareholder value by requiring executives to hold Stevanato Group common stock over the long term. The Compensation Committee is responsible for monitoring compliance with these guidelines on an annual basis.
101
The Chief Executive Officer is required to hold at least five times his/ her base salary while the other senior officers two times their respective base salary. Executives are expected to comply with the applicable guidelines by the fifth anniversary of the date in which they after first become subject to the guidelines, and they are expected to continue to own sufficient shares to comply with the guidelines at all times following such date.
As a “foreign private issuer,” as defined by the SEC, we are permitted to follow home country corporate governance practices instead of certain corporate governance practices required by NYSE applicable to U.S. domestic issuers.
If we cease to be a “foreign private issuer” under the NYSE rules and the Exchange Act, as applicable, we will take all action necessary to comply with applicable NYSE corporate governance rules.
Because we are a foreign private issuer, our directors and senior management are not subject to short-swing profit and insider trading reporting obligations under Section 16 of the Exchange Act. They will, however, be subject to the obligations to report changes in share ownership under Section 13 of the Exchange Act and related SEC rules.
The provisions of the Italian Civil Code regulating companies that are listed on a regulated market (societa’ che fanno ricorso al mercato di capitale di rischio) apply to the Company. As described in more detail below, these rules differ in a number of ways from those applicable to U.S. domestic companies under NYSE listing standards, as set forth in the NYSE Listed Company Manual.
Board of Directors
The Italian Civil Code provides for three alternative corporate governance systems: (i) the traditional model (comprising a board of directors and a board of statutory auditors), (ii) the two-tier board system (comprising a management board and a supervisory board) or (iii) the one-tier board system (comprising a board of directors and an Audit Committee).
In May 2021, we adopted the one-tier corporate governance system, which provides for a Board of Directors and an Audit Committee. The board of directors is appointed by the shareholders’ meeting and the Audit Committee is, in turn, appointed by the board of directors from among its members (as appointed by the shareholders’ meeting).
The board of directors is generally responsible for managing the affairs of the company. The Board may therefore undertake all transactions considered necessary, useful or appropriate in achieving the company’s corporate purpose except only for such actions as are reserved to the ordinary or extraordinary shareholders’ meeting by applicable law or the articles of association.
Within the limits prescribed by Italian Law, the Board may delegate its general powers to an executive committee and/or managing director to handle the day-to-day management consistent with the guidelines set by the board of directors. The Chairman of the board of directors, any deputy chairman as well as any managing director are authorized to represent and bind the company in their capacity as legal representatives. The board of directors and any managing director may also delegate the power to carry out certain acts within the scope of their respective authority.
Our board of directors consists of 12 directors (including the members of the Audit Committee) and has been appointed by the ordinary shareholders’ meeting on May 28, 2021 for a period of three fiscal years. A new Board of Directors will be appointed in May of 2024. Members of the board of directors who are also employees are entitled to applicable severance pay benefits (TFR) under Italian law. No other service contracts and/or agreements exist between members of the board of directors, us and/or our subsidiaries, providing for benefits and/or compensation to our directors upon termination of employment.
During 2023, the Board of Directors has been convened n. 9 times.
Foreign Private Issuer Status
As a foreign private issuer whose shares are listed on the NYSE, we have the option to follow certain Italian corporate governance practices rather than those of NYSE, except to the extent that such laws would be contrary to
102
U.S. securities laws and provided that we disclose the practices we are not following and describe the home country practices we are following. We rely on this “foreign private issuer exemption” with respect to the following NYSE Corporate Governance Standards:
Except as stated above, we comply with the rules generally applicable to U.S. domestic companies listed on NYSE. We may in the future decide to use other foreign private issuer exemptions with respect to some or all of the other NYSE listing requirements. Following our home country governance practices, as opposed to the requirements that would otherwise apply to a company listed on NYSE, may provide less protection than is accorded to investors under NYSE listing requirements applicable to domestic issuers. For more information, see “Risk Factors—Risks Relating to our Initial Public Offering and Ownership of our Shares—As we are a “foreign private issuer” and intend to follow certain home country corporate governance practices, our shareholders may not have the same protections afforded to shareholders of companies that are subject to all NYSE corporate governance requirements.”
Committees of the Board of Directors
On May 28, 2021, we established an Audit Committee while on June 16, 2021, we established a Compensation Committee, a Nomination and Corporate Governance Committee, a ESG Committee and a Business and Strategy Committee. Each of these committees are governed by a charter that is consistent with applicable Italian Law and SEC and NYSE corporate governance rules, and which is available on the Investors section of our website at https://www.stevanatogroup.com/en/. The information contained on, or that can be accessed through, our website does not form part of this annual report.
Audit Committee
Our Audit Committee consists of William Federici, Fabio Buttignon and Fabrizio Bonanni. Mr. Federici serves as the chairman of the Audit Committee. Our board determined that all members of our Audit Committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and the NYSE corporate governance rules. Our board determined that Fabio Buttignon and William Federici are both audit committee financial experts as defined by the SEC rules and have the requisite financial experience as defined by the NYSE corporate governance rules. Further, Mr. Buttignon is a certified accountant and, in such capacity, is enrolled with the Italian Registry of Statutory Auditors.
Our board determined that each member of our Audit Committee is “independent” as such term is defined under Italian Law, it being understood that a director cannot qualify as independent (and, therefore, cannot be an Audit Committee member) if any of the following applies: (i) being interdict, incapacitated, bankrupt, or convicted of an offense that implies the interdiction, even temporary, from public offices, or the inability to exercise managerial offices; (ii) being the spouse, relatives and relatives-in-law within the fourth degree of directors of the company, the directors themselves, the spouse, relatives and relatives-in-law within the fourth degree of directors of the companies controlled by the concerned company, of the companies that control it and of those subject to common control; and (iii) being linked to the company or to the companies controlled by it or to the companies that control it or to those subject to common control by an employment relationship or by an ongoing relationship of consultancy or paid work, or by other relationships of a financial nature that compromise their independence.
Our Audit Committee is compliant with applicable rules and regulations of the SEC and NYSE corporate governance rules as well as Italian Law requirements with respect to its composition, expertise requisites and functioning.
103
The Audit Committee is responsible for, among other things, assisting the board in the oversight of:
The Audit Committee meets regularly and in a manner that the Audit Committee may deem fit and, at least once every ninety calendar days. Periodically, the Audit Committee also meets with our independent auditor and members of our management.
During 2023, the Audit Committee has been convened n. 8 times.
Compensation Committee
Although not required under Italian law, on June 16, 2021, we established a compensation committee. This committee consists of Madhavan Balachandran (as chairman), Donald Eugene Morel Jr. and Paola Vezzaro.
The Compensation Committee is responsible for, among other things:
Pursuant to Italian Law, the shareholders’ meeting determines the base compensation of the members of the board of directors. After consultation with the Audit Committee, the board of directors may determine the compensation of executive officers, including the CEO. If the articles of association so provide, the shareholders’ meeting may determine an aggregate amount for the remuneration of all directors, including executive officers.
During 2023, the Compensation Committee has been convened n. 6 times.
Nomination and Corporate Governance Committee
Although not required under Italian law, on June 16, 2021, we established a nomination and corporate governance committee. This committee consists of Franco Stevanato (as chairman), Madhavan Balachandran, Donald Eugene Morel Jr. and Fabrizio Bonanni.
The Nomination and Corporate Governance Committee is responsible for, among other things:
104
If, during the term of their office, one or more directors resign, the other directors must replace them by a resolution approved by the Audit Committee, provided that the majority of the board still comprises directors appointed by the company’s shareholders. The coopted directors remain in office until the next shareholders’ meeting. If at any time more than half of the members of the board of directors appointed by the shareholders’ meeting resign, the remaining members of the board of directors (or the Audit Committee if all the members of the board of directors have resigned or ceased to be directors) must promptly call an ordinary shareholders’ meeting to appoint the new directors and until such time as the new directors are appointed, the resigning directors remain in office.
During 2023, the Nomination and Corporate Governance Committee has been convened n. 3 times.
Business & Strategy Committee
On June 16, 2021, we established a Business and Strategy Committee. This committee consists of Donald Eugene Morel Jr. (as chairman), Sergio Stevanato, Franco Stevanato, Marco Stevanato, Madhavan Balachandran, Fabrizio Bonanni, Franco Moro and William Federici.
The Business and Strategy Committee is responsible for, among other things:
During 2023, the Business & Strategy Committee has been convened n. 5 times.
ESG Committee
On June 16, 2021, we established a ESG Committee. Our ESG Committee consists of Fabiano Nicoletti (as chairman), Paola Vezzaro and William Federici.
The ESG Committee is responsible for, among other things:
105
During 2023, the ESG Committee has been convened n. 4 times.
Duties of Directors and Conflict of Interests
Under Italian law, the primary duty of directors is to carry out all activities as are necessary for the achievement of the corporate purpose in accordance with applicable law and the articles of association.
In particular, directors have a general duty to act with care, without self-interest and on a well-informed basis.
The applicable standard of conduct is determined, on a case-by-case basis, taking into account the characteristics of the corporation, the specific tasks and responsibilities conferred to the single directors, and the personal skills of the latter.
In addition, directors have numerous specific duties and obligations, such as, inter alia:—keeping the corporation’s books, records and other databases (including the shareholders’ register) in such a manner that the corporation’s rights and obligations may be ascertained from the interested parties at all times;—preparing the corporation’s annual accounts according with the applicable accounting principles and filing them with the Companies’ Register on time;—registering the corporation with the Companies’ Register and keeping the registered information up to date;—convening annually or when necessary or required by the shareholders the general meetings of the corporation; and—monitoring the own funds and financial position of the corporation and initiate the actions or procedures contemplated by the law in case of (i) losses entailing the reduction of the own funds of the corporation below the threshold of two thirds of the share capital or (ii) income, asset or financial unbalances having certain characteristics.
The board of directors may delegate certain powers to one or more managing directors (amministratori delegati), determine the nature and scope of the powers delegated to each director and revoke such delegation at any time. The managing directors must report to the board of directors and the Audit Committee at least every 180 days on the company’s business and the main transactions carried out by the company or by its subsidiaries.
Directors having any interest in a proposed transaction must disclose such interest to the board of directors and to the Audit Committee, even if such interest is not (or is deemed not to be) in conflict with the interest of the company in the same transaction. The interested director is not required to abstain from voting on the resolution approving the transaction, but the resolution must state explicitly the reasons for, and the benefit to the company of, the approved transaction. In the event that these provisions are not complied with, or that the transaction would not have been approved but for the vote of the interested director, the resolution may be challenged by a director or by the Audit Committee if the approved transaction is (or is likely to be) prejudicial to the company. If the director carrying an interest in the transaction is the CEO and the transaction falls within his/her competence, he/she will in any case have to abstain from carrying out the transaction on behalf of the Company and will defer authority to the board of directors.
Terms of Directors and Officers
The board of directors is elected by the ordinary shareholders’ meeting of the Company, for the period established at the time of election but in any event for no more than three fiscal years. A director may be reappointed for successive terms.
The board of directors may also appoint one or more general managers (direttori generali), who must report directly to the board of directors and confer powers for single acts or categories of acts to employees of the company or third-party representatives.
Under Italian law and pursuant to our articles of association, directors may be removed from office at any time by the shareholders’ meeting. A director that is removed without cause may have a claim for damages against the Company. Directors may resign at any time by written notice to the board of directors and to the chairman of the Audit
106
Committee. The board of directors, subject to the approval of the Audit Committee, must appoint substitute directors to fill vacancies arising from removals or resignations to serve until the next ordinary shareholders’ meeting.
If at any time more than half of the members of the board of directors appointed by the shareholders’ meeting of the Company resign, the remaining members of the board of directors (or the Audit Committee if all the members of the board of directors have resigned or ceased to be directors) must promptly call an ordinary shareholders’ meeting to appoint the new directors and until such time as the new directors are appointed, the resigning directors remain in office.
Please see the section entitled “Item 4. Information on the Company—B. Business Overview—Our Business—Employees” for more information concerning the number of our employees and related information.
Please see the sections entitled “—B. Compensation—Stock Grant Plan” and “Item 7. Major Shareholders and Related Party Transactions—A. Major Shareholders” for more information concerning our arrangements for involving employees in the capital of the company and the share ownership of our directors and executive officers, respectively.
F. DISCLOSURE OF A REGISTRANT'S ACTION TO RECOVER ERRONEOUSLY AWARDED COMPENSATION
None.
Except as specifically noted, the following table sets forth information with respect to the beneficial ownership of our shares as of the date of this annual report as adjusted to reflect the ownership of our Class A and ordinary shares in for:
As of the date of this annual report, our share capital comprised 295,540,036 shares (including 30,073,093 Class A shares held by the Company in treasury), including two shareholders of record holding Class A shares (being Stevanato Holding S.r.l. holding 230,596,476 Class A shares, and the Company holding 30,073,093 Class A shares in treasury) and shareholders of record holding ordinary shares (for an aggregate of 34,870,467 ordinary shares). Stevanato Holding S.r.l. holds approximately, respectively, 78.03% and 95.20% of our share capital and of the voting rights (excluding treasury shares), while other shareholders (excluding the Company) hold in aggregate approximately, respectively, 11.80% and 4.80% of our share capital and of the voting rights (excluding treasury shares). The latter percentages are not representative of the portion of our shares held in the United States nor are they representative of the number of beneficial holders residing in the United States, and are mostly held beneficially from undisclosed shareholders.
The dual class structure of our shares includes ordinary shares and Class A shares. Holders of our ordinary shares have the same voting rights and are entitled to one vote per share, while holders of Class A shares (held solely by Stevanato Holding S.r.l. or in treasury by the Company) are entitled to three votes per share.
Unless otherwise indicated below, the address for each beneficial owner listed is Via Molinella, no. 17, Padua, Piombino Dese, Italy.
107
Beneficial Owner (Name/Address) |
|
Ordinary shares owned |
|
|
Class A shares owned |
|
|
Percentage of voting rights |
|
|||
Stevanato Holding S.r.l.(1) |
|
|
— |
|
|
|
230,596,476 |
|
|
|
95.20 |
% |
Stevanato Group S.p.A. |
|
|
— |
|
|
|
30,073,093 |
|
|
|
— |
|
Directors and Executive Officers: |
|
|
|
|
|
|
|
|
|
|||
Sergio Stevanato |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Franco Stevanato |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Marco Stevanato |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Fabiano Nicoletti |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Alvise Spinazzi |
|
|
3,300 |
|
|
|
— |
|
|
* |
|
|
Fabrizio Bonanni |
|
|
146,633 |
|
|
|
— |
|
|
* |
|
|
Fabio Buttignon |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Madhavan Balachandran |
|
|
58,103 |
|
|
|
— |
|
|
* |
|
|
Donald Jr Eugene Morel |
|
|
46,069 |
|
|
|
— |
|
|
* |
|
|
William Federici |
|
|
47,225 |
|
|
|
— |
|
|
* |
|
|
Paola Vezzaro |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Franco Moro |
|
|
123,813 |
|
|
|
— |
|
|
* |
|
|
Marco Dal Lago |
|
|
81,654 |
|
|
|
— |
|
|
* |
|
|
Mauro Stocchi |
|
|
412,888 |
|
|
|
— |
|
|
* |
|
|
Paolo Patri |
|
|
92,288 |
|
|
|
— |
|
|
* |
|
|
Riccardo Butta |
|
|
— |
|
|
|
— |
|
|
|
— |
|
All Directors and Executive Officers as a Group (15 persons) |
|
|
1,011,973 |
|
|
|
— |
|
|
* |
|
|
* Less than 1% of voting rights as of the date of this annual report.
Our Board adopted a written statement of policy for the evaluation of and the approval, disapproval and monitoring of transactions involving us and “related persons.” For the purposes of the policy, “related persons” include our executive officers, directors, director nominees, and shareholders owning five percent or more of our outstanding shares, and each of their respective immediate family members.
The policy covers any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we, or any of our parent or subsidiary companies, were or are to be a participant, and which are unusual in their nature or conditions, involving goods, services or tangible or intangible assets.
Pursuant to this policy, our management presents to our Audit Committee each proposed related person transaction, including all relevant facts and circumstances relating thereto. Our Audit Committee then:
108
All related person transactions may only be consummated if our Audit Committee has approved or ratified such transaction in accordance with the guidelines set forth in the policy. Certain types of transactions have been pre-approved by our Audit Committee under the policy. These pre-approved transactions include:
No director may participate in the approval of a related person transaction for which he or she, or his or her immediate family members, is a related person. In the event that an insufficient number of members of the Audit Committee is disinterested with regard to a specific transaction to achieve a quorum, such transaction will be considered by the members of the Board that are disinterested with regard to such transaction.
Within this section, we have calculated the U.S. Dollar amounts using the historical exchange rate as of the date of each transaction. Other than compensation arrangements described in “Management” elsewhere in this annual report, since January 1, 2018, we have engaged in the following material transactions with our executive officers, directors or holders of more than 5% of our share capital, including their affiliates, which we refer to as our related parties. Stevanato Holding S.r.l. is currently our largest shareholder. Stevanato Holding S.r.l. owns 230,596,476 of our Class A shares, which will represent approximately 78.03% of our shares outstanding. For more information, see "Item 7. Major Shareholders and Related Party Transactions—A. Major Shareholders".
Payment of Service Fees and Rentals
During the years ended December 31, 2021, 2022, and 2023 Ompi of Japan disbursed €352,341, and €312,641, €190,743 respectively, to Winckler & Co., Ltd. in connection with the rental of offices and warehouses and the supply of corporate services. Winckler & Co., Ltd. held a 49% stake in Ompi of Japan until July 31, 2023 when Stevanato Group S.p.A. purchased the remaining minority equity interests.
Payment of Consultancy Fees
We have an ongoing professional relationship with Studio Legale SAT, pursuant to which Studio Legale SAT provides legal services to the Company from time to time. In connection with these services, we paid € 578,199, €383,980 and €310,970 during the years ended December 31, 2021, 2022, and 2023 respectively, to Studio Legale SAT. Mr. Alvise Spinazzi, member of the board of Stevanato Group, is a Partner of Studio Legale SAT.
Payment of Consultancy Fees
On June 7, 2017, we entered into a consulting agreement with MJB Consultants LLC, pursuant to which we agreed to pay MJB Consultants LLC a fee for consulting and legal services provided to us and our subsidiaries. Pursuant to this agreement, we paid €56,791 for the year ended December 31, 2021. Mr. Madhavan Balachandran, member of the board of Stevanato Group, is the beneficial owner of MJB Consultants LLC.
109
Payment of Consultancy Fees
We have a consulting relationship with Progenitor Capital Partners LLC, pursuant to which we pay Progenitor Capital Partners LLC a fee for consulting and legal services provided to us and our subsidiaries. Pursuant to this relationship, we paid €67,128 for the year ended December 31, 2021. Dr. Don Morel, member of the board of Stevanato Group, is a beneficial owner of Progenitor Capital Partners LLC.
Industrial Rent
In the years 2021, 2022, and 2023 SVM Automatik (a subsidiary of Stevanato Group) disbursed €410,444, €418,714 and € 434,798 respectively, to E & FKH Ejendomme ApS in connection with the rental of the plant where Stevanato Group Denmark operates. The beneficial owners of E & FKH Ejendomme ApS are family members of a board member in Stevanato Group Denmark.
Payments in connection with Rent
For each of 2021, 2022, and 2023 the Company recognized costs for €18,755, €19,309, and €20,421 respectively to SFEM Italia S.r.l. in connection with certain rental installments. SFEM Italia S.r.l. is controlled by Sergio Stevanato, Franco Stevanato and Marco Stevanato, each members of the Stevanato family.
Drug Containment Solutions Revenues
In the year 2021 the Group sold Drug Containment Solutions to SwissFillon AG, a Swiss filling company start-up, for a total amount of €564,790. Stevanato Group S.p.A. held a 27% stake in SwissFillon AG up to October 22, 2021 when the Company sold this minority interest.
During the fiscal year ended December 31, 2021, 2022 and 2023 the Group supplied Drug Containment Solutions to Incog BioPharma Services, Inc. (“Incog”), a U.S. based biopharma services company, for a total amount of €670,635, €508,937 and €544,534 respectively. Incog is majority owned by SFEM Italia S.r.l..
Donations to Stevanato Foundation
In the years 2021, 2022, and 2023 we made aggregate donations to the Stevanato Foundation of €180,000, €305,000 and €240,000 respectively. The Stevanato Foundation is a charitable organization entirely owned by the Stevanato Family. The Stevanato Foundation exclusively pursues aims of social solidarity, philanthropy and charity, operating in the fields of social and socio-medical assistance, education and training, as well as cultural and educational activities and scientific research. A key function of the Stevanato Foundation is operating in support of the health, education, and maintenance of children and young people who are in strenuous conditions due to health, financial or other reasons.
Land purchase
In the year 2023 we entered in a promissory agreement for the purchase of land in Mexico with SIT Manufacturing N.A. S.A. de C.V. and paid $ 2,247,254 as a deposit. On January 16, 2024 the purchase of the land was officially concluded with the payment of the remaining $ 1,210,061. Franco Stevanato is a Board member in SIT S.p.A., the parent company of SIT Manufacturing N.A. S.A. de C.V.
Loan in Connection with Stock Grant Plan
On July 16, 2021, the Company disbursed an aggregate of €443,636 as loans to Mr. Marco Dal Lago and Mr. Paolo
Patri to facilitate both individuals in paying tax due in respect of income represented by the stocks granted in connection with the 2021-2027 Stock Grant Plan. Mr. Marco Dal Lago is the Chief Financial Officer of the Company
and Mr. Paolo Patri was the Chief Technology Officer of the Company. At the time the loans were made, management believed that these loans were permissible and did not violate Section 13(k) of the Exchange Act. Upon being advised that such loans were impermissible, the two executives repaid such loans in full, after the end of the first quarter 2022. As a result of this inadvertent violation, the Board adopted a policy regarding loans or advances to any Executive Officer or Director of the Company. The policy provides that "The Company shall not directly or indirectly, including
110
through any subsidiary, extend or maintain credit to, or arrange for the extension of credit, or renew an extension of credit, in the form of a personal loan to or for any Director or Executive Officer (or equivalent thereof) of the Company or any subsidiary of the Company."
Share-based Awards to Directors and Executive Management
We have granted share-based awards to certain of our directors and executive management. For more information regarding the warrants granted to our executive management and directors see the section herein entitled “Management—Stock Grant Plan.”
Indemnification Agreements
In connection with our listing on the NYSE, we entered into indemnification agreements with our directors and executive officers. These indemnification agreements require us to indemnify our directors and executive officers to the fullest extent permitted by law, save for a limited number of instances, including when (i) officers and directors’ acts or omissions constituted willful misconduct or gross negligence, (ii) officers and directors did not act in good faith, for a purpose which they reasonably believed to be in, or not opposed to, the best interests of the Company and (iii) officers and directors are held liable towards the Company.
Insofar as indemnification of liabilities arising under the Securities Act may be permitted to executive officers and board members or persons controlling us pursuant to the foregoing provisions, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Registration Rights Agreement
Upon completion of our initial public offering on the NYSE, we and certain of our then existing shareholders entered a the Registration Rights Agreement. The Registration Rights Agreement provides to such shareholders certain registration rights relating to our ordinary shares held by them, subject to customary restrictions and exceptions. Registration of such registrable securities would result in registration of ordinary shares under the Securities Act and would result in these shares becoming freely tradable without restriction under the Securities Act immediately upon the effectiveness of the registration, except for shares purchased by affiliates.
Not applicable.
111
ITEM 8. Financial Information
Please see the section entitled “Item 18. Financial Statements” for more information on the financial statements filed as a part of this annual report. Please also see the section entitled “Item 4. Information on the Company—B. Business Overview—Our Business” for a discussion of legal proceedings and the section entitled “Item 10. Additional Information—B. Memorandum of Association and By-Laws” for a description of our dividend policy.
Please see the section entitled “Item 5. Operating and Financial Review and Prospects” for more information concerning for information concerning any significant changes that may have occurred since the date of our annual financial statements.
ITEM 9. The Offer and Listing
Our Shares are listed on the New York Stock Exchange, our principal host market, under the symbol “STVN”.
Not applicable.
See “—Offer and Listing Details” above.
Not applicable.
Not applicable.
Not applicable.
ITEM 10. Additional Information
The following is a summary of certain information concerning our shares and certain provisions of our articles of association and of Italian law insofar as they relate to our ordinary shares. It may not contain all of the information that may be relevant to an investor in deciding whether to invest in the ordinary shares. This summary is qualified in its entirety by reference to our articles of association and applicable Italian law. Prospective investors are urged to read the complete form of our articles of association which have been filed with the SEC as an exhibit to our annual report of which this annual report is a part.
We are a joint stock company (società per azioni) incorporated in the Republic of Italy and our corporate affairs are governed by our articles of association, certain provisions of the Italian Civil Code, which we refer to as the Civil Code below, and the laws of the Republic of Italy.
Our authorized share capital is €21,698,480.00 divided into 295,540,036 shares without par value, broken down as follows: (a) 34,870,467 are ordinary shares; and (b) 260,669,569 Class A shares of which 30,073,093 Class A shares held in treasury (the ordinary shares together with the Class A shares, the “shares”). All of our issued and outstanding shares are fully paid. We have 265,466,943 shares outstanding (not including 30,073,093 Class A shares held in treasury).
112
We did not enter any agreement or other undertaking to increase the share capital.
Over the past three years, our share capital was increased from €20,002,000.00 to €21,698,480.00, while the number of issued shares was increased from 20,002 ordinary shares, carrying a single voting right per share, to total 295,540,036 shares belonging to different share classes, the rights attached to which are described in the Section B below. In particular, our share capital changed as follows:
- on March 4, 2021, the shareholders’ meeting approved a share split following which the then existing 20,002 shares have been split into a total of 100,010,000 ordinary shares, without changing the amount of the share capital, then equal to €20,002,000.00;
- on July 1, 2021, the shareholders’ meeting approved (i) a further share split following which all the existing 100,010,000 shares have been split into a total of 272,427,240 shares in the ratio of 2,724 new shares post-split for each share outstanding prior to the share split and (ii) a divisible share capital increase for a maximum nominal amount of €2,936,857.00, by issuance of maximum no. 40,000,000 new ordinary shares to be offered to the underwriters in the context of our initial public offering;
- in connection with the split that occurred on July 1, 2021, all of the ordinary shares held by Stevanato Holding S.r.l. and the ordinary shares held in treasury were converted into Class A shares;
- on July 20, 2021, following completion of our initial public offering, our share capital was increased by the nominal amount of €1,644,160.00 by issuance of 22,400,000 ordinary shares; as a result of such share capital increase and of the sale of 9,600,000 ordinary shares (post conversion) by Stevanato Holding S.r.l. to the underwriters, our share capital was €21,646,160 and divided into total 294,827,240 shares, including 33,084,725 ordinary shares and 261,742,515 Class A shares;
- on August 18, 2021, following the exercise by the underwriters of the over-allotment option, our share capital was increased by the nominal amount of €52,320 by issuance of 712,796 ordinary shares; as a result of such share capital increase and of the sale of 305,484 ordinary shares (post conversion) by Stevanato Holding S.r.l. to the underwriters, our share capital was €21,698,480.00 and divided into total 295,540,036 shares, including 34,103,005 ordinary shares and 261,437,031 Class A shares;
- on August 30, 2023, the Company granted 364,139 ordinary shares (post conversion) previously held in treasury to the beneficiaries of the Restricted Stock Grant Plan Stevanato Group S.p.A. 2021-2022; as a result of such granting, the share capital was divided into total 295,540,036 shares, including 34,467,144 ordinary shares and 261,072,892 Class A shares;
- on September 7, 2023, the Company granted 403,323 ordinary shares (post conversion) previously held in treasury to certain directors and employers of the Company or of its subsidiaries; as a result of such granting, the share capital was divided into total 295,540,036 shares, including 34,870,467 ordinary shares and 260,669,569 Class A shares; and
- on October 4, 2023, in the Company’s extraordinary shareholders’ meeting the shareholders voted to delegate to the board of directors, pursuant to article 2443 of the Italian Civil Code, the authority to increase the share capital in cash, on one or more occasions, also on a divisible basis pursuant to article 2439 of the Italian Civil Code, within the term of October 4, 2028, for a maximum amount of €350,000,000.00, including any share premium, by issuing ordinary shares, with no par value, carrying full dividend rights, in one or more tranches, to be offered by excluding the existing shareholders’ pre-emptive right pursuant to article 2441, paragraph 4, second sentence, of the Italian Civil Code (and, therefore, within the limit of 10% (ten per cent) of the overall number of Company’s shares currently outstanding). The newly issued ordinary shares resulting from such share capital increases resolved upon by the board of directors shall be offered to persons falling into one of the following categories:
113
B. Memorandum of Association and By-Laws
The following are summaries of material provisions of our articles of association, insofar as they relate to the material terms of our shares.
Objects of Our Company.
Our corporate purpose consists of:
The activities referred to in points (i) and (ii) above shall not be carried out vis-à-vis the public. In particular, all activities concerning trusts, collection of savings, exercise of credit, placement on the market of financial instruments are excluded, together with all other activities Italian law reserves for specific entities.
Applicable provisions
With its ordinary shares listed on the NYSE, the Company is subject to the provisions of the Italian Civil Code applicable to companies that are listed on a regulated market (società che fanno ricorso al mercato del capitale di rischio).
Form, Transfer of Shares and Voting Rights.
The share capital of the Company is equal to €21,698,480.00 and is divided into 295,540,036 shares, broken down as follows: (a) 34,870,467 ordinary shares; and (b) 260,669,569 Class A shares.
The share capital can also be increased by means of contributions in kind (including receivables) and by issuing different classes of shares, in compliance with the applicable provisions of law and of the articles of association.
The extraordinary shareholders’ meeting may grant the board of directors, pursuant to article 2443 of the Italian Civil Code, the power to increase the Company’s share capital, on one or more occasions, up to a certain amount and for a maximum period of five years from the date of the relevant resolution, as well as the power to issue convertible bonds, up to a certain amount and for a maximum period of five years from the date of the relevant resolution.
114
The shareholders’ pre-emption right on the newly issued ordinary shares may be excluded, pursuant to article 2441, paragraph 4, second sentence, of the Italian Civil Code, within the limit of 10 percent of the pre-existing share capital, provided that the issue price is equal to the market value of the ordinary shares and this is confirmed by a specific report by a statutory auditing firm or auditor.
The Company may also issue bonds and equity, as well as non-interest-bearing financial instruments, convertible or non-convertible into shares, warrants and other financial instruments in compliance with the applicable provisions of law and of the articles of association. The shares are not issued in form of share certificates, pursuant to article 2346, paragraph 1 of the Italian Civil Code, and are not subject to the dematerialization regime (regime di dematerializzazione) pursuant to article 83-bis et seq. of the Italian Financial Act (Testo Unico della Finanza). The exercise of shareholders’ rights is subject to the provisions of article 2355, paragraph 1, of the Italian Civil Code, unless otherwise provided in the articles of association.
The Company keeps the shareholders’ register (libro soci), in paper form or electronically, in accordance with the provisions of article 2215-bis of the Italian Civil Code and with the laws and regulations in force (the “Shareholders’ Register”).
The ordinary shares shall be transferred on the basis of the documentation or of the IT techniques customarily used by NYSE operators, in accordance with the U.S. laws and regulations and with the NYSE listing rules.
The board of directors shall be entitled to establish and maintain, by appointing a third-party company authorized to provide “transfer agency” services in relation to financial instruments traded on the NYSE and supervised by the competent U.S. Authorities (the “Transfer Agent & Registrar”), a paper and/or electronic register, in compliance with U.S. regulations (the “US Register”), in which the parties that hold direct ownership of ordinary shares and the related share transfers are recorded, with a subsequent corresponding entry in the Shareholders’ Register (the “Registered Shareholders”). As long as the U.S. Register is established, as a result of the trading of ordinary shares on the NYSE and only with respect to such shares, the registration of transfers in the U.S. Register constitutes a prerequisite for the regularity and validity of the subsequent corresponding entries in the Shareholders’ Register, without prejudice to the legal nature and relevance of the latter pursuant to Italian law.
The board of directors is entitled to establish procedures, by appointing a third-party providers or otherwise, for the identification of the persons who - as a consequence of the registration in the U.S. Register pursuant to applicable regulations of a single depository entity of the ordinary shares (the “Holder of Record”), as designated by the company responsible for the centralized management - hold indirect ownership of the ordinary shares (the “Beneficial Owners”) and are therefore entitled to indirectly exercise the corporate rights pertaining to them.
Only with respect to the ordinary shares:
It is understood that the exercise of corporate rights by the Beneficial Owners, in the name of the Holder of Record, both collectively and individually, does not entail any obligation to update the U.S. Register and the Shareholders’ Register.
Ordinary shares are registered, indivisible, freely transferable and give their holders equal rights. In particular, each ordinary share grants the right to one vote at the ordinary and extraordinary shareholders’ meetings of the Company and the other administrative rights pertaining to shareholders pursuant to the law and the articles of association.
115
The Class A shares have the same characteristics and grant the shareholders the same rights as the ordinary shares, except that:
Under no circumstances may ordinary shares be converted into Class A shares.
The Company may issue Class A shares only in the event of: (a) capital increase by means of new cash contributions without exclusion or limitation of pre-emption rights; (b) capital increases without new contributions pursuant to article 2442 of the Italian Civil Code; and (c) mergers or spin-offs, in any event in combination with ordinary shares.
In the event of a share capital increase without exclusion of pre-emptive rights to be carried out through the issue of ordinary shares only, the right to subscribe to the newly issued ordinary shares shall be granted pro-rata to all shareholders in proportion to the number of shares (whether ordinary shares or Class A shares) held by each of them at the time of execution of the share capital increase.
In the event of a share capital increase by way of issuance of ordinary shares and Class A shares: (a) the percentage of newly issued ordinary shares and Class A shares shall reflect the same proportion between ordinary shares and Class A shares as that current at the date of the relevant resolution; and (b) the newly issued ordinary shares and Class A shares shall be offered for subscription to the individual shareholders pro-rata to the number of ordinary shares and Class A shares held by each of them at the time of execution of the capital increase, it being understood that if any Class A shares remain unsubscribed by holders of Class A shares at the end of the subscription period, pursuant to article 2441, paragraph 2, of the Italian Civil Code, such Class A shares may be subscribed as ordinary shares by parties other than the holders of Class A shares.
In the event the Company takes part in a merger or demerger transaction, the holders of Class A shares will be entitled to receive, in exchange for or in addition to the Class A shares held by them, shares having the same characteristics as the Class A shares, so far as legally permitted, unless otherwise resolved by a special meeting of the shareholders holding the Class A shares.
Dividends
Payment of any annual dividends by the Company may be made out of its distributable profits and reserves for each relevant year by resolution of the shareholders’ meeting.
The payment of annual dividends is proposed by the board of directors and is subject to the approval by the shareholders at the annual general meeting. Before dividends are paid out, an amount equal to five percent of net distributable profits shall be allocated to the Company’s legal reserve until such reserve is equal to at least one fifth of the nominal value of the Company’s issued share capital.
116
If the Company’s own funds are reduced to an amount lower than the Company’s share capital as a result of losses, dividends may not be paid until the share capital is reconstituted or reduced by the same amount as the existing own funds. If the conditions provided for by article 2433-bis of the Italian Civil Code are met, the board of directors may authorize, during the course of the financial year, the distribution of interim dividends, subject to certain limitations. The payment of dividends or interim dividends and other distributions to the shareholders shall be made within the terms and in the manner determined by the shareholders’ meeting or the board of directors that took the relevant resolution.
The board of directors shall determine the relevant date for identifying the Beneficial Owners entitled to receive payment of dividends, other distributions or assignments of rights to the shares held by the Holder of Record. Such date may be set at the same time as, before, or after, the date on which the dividend payment, distribution or assignment is resolved by the ordinary shareholders’ meeting or the board of directors.
Shareholders’ Meetings
Shareholders are entitled to attend and vote at shareholders’ meetings, provided that they are registered on the U.S. Register and the Shareholders’ Register as of the end (on New York’s time zone) of the 25th day prior to each shareholders’ meeting or, in case such day is not a trading day, on the preceding day (the “Record Date”). Shareholders remain entitled to intervene and vote at the shareholders’ meetings even if they have transferred their shares after the Record Date. Moreover, the shareholders who are registered as such on the U.S. Register and the Shareholders’ Register after the Record Date but before the general meeting’s date are deemed not to have attended or voted in favor of the resolutions passed by such meetings for the purposes of challenging the resolutions or exercising the right of withdrawal pursuant to articles 2377 and 2437 of the Italian Civil Code, respectively. It is understood that the Beneficial Owners who were such as of the Record Date and have obtained registration in both the US Register and the Shareholders’ Register between the Record Date and the date of the Shareholders’ Meeting, will be able to challenge the resolutions and exercise the right to withdraw pursuant to articles 2377 and 2437 of the Italian Civil Code, only by proving that they were Beneficial Owners of the shares at the time of the adoption of the relevant resolutions and did not vote in favor of such resolutions.
The shareholders’ meeting is convened by the board of directors, and may be held in a place other than the registered office, in Italy, in other countries of the European Union, in the United Kingdom or in the United States of America.
The board of directors shall call the shareholders’ meeting without delay when it is requested to do so by a number of shareholders representing at least five per cent of the share capital of the Company pursuant to article 2367 of the Italian Civil Code.
The shareholders’ meeting, whether ordinary or extraordinary, shall be held on first call and, if necessary, on second call, as well as on subsequent calls, unless the board of directors establishes in the Notice of Call (as defined below) that the shareholders’ meeting shall be held in a single call.
Ordinary and extraordinary shareholders’ meetings must be convened by means of a notice to be published, in the manner specified below, at least 40 days prior to the date of the meeting (the “Notice of Call”).
The Notice of Call shall be published:
The Notice of Call shall contain:
117
The shareholders’ meeting is chaired by the Chairman of the board of directors or, in case of absence or impediment, in order, by a vice-chairman, by a managing director, if appointed, or, in case of absence or impediment of the latter, by another person appointed by the shareholders’ meeting by majority vote of those present.
The chairman of the shareholders’ meeting is assisted by a secretary, who may or may not be a shareholder, appointed by the shareholders’ meeting itself upon proposal of the chairman with the majority vote of those present. In extraordinary shareholders’ meetings and, in any case, when the chairman deems it appropriate.
The chairman of the shareholders’ meeting ascertains the identity and the right to intervene of those attending the meeting, verifies that the shareholders’ meeting has been duly constituted, regulates its proceedings, establishes the voting procedures in accordance with applicable law and ascertains the results of voting.
Minutes of the shareholders’ meeting must be drawn up in accordance with applicable law, signed by the chairman of the meeting and by the secretary or notary, and subsequently copied in the book of the meetings and resolutions of the shareholders’ meeting.
Ordinary Shareholders’ Meeting
The ordinary shareholders’ meeting may resolve upon all matters reserved to it by applicable law and by the articles of association.
The ordinary shareholders’ meeting is validly constituted and approves resolutions in first, second and any subsequent calls or, if so established in the Notice of Call, in a single call, with the quorums required by applicable law. For the purpose of calculating the applicable quorums, the number of votes pertaining to the shares and not the number of shares is taken into account.
In first call, the ordinary shareholders’ meeting is duly held with the presence of shareholders representing the majority of the overall votes relating to the shares issued by the Company, and approves resolutions with the absolute majority (maggioranza assoluta) of the overall votes relating to the shares held by the shareholders attending the meeting.
In second call, in subsequent calls or in a single call, the ordinary shareholders’ meeting is duly held regardless of the number of votes represented by the shareholders attending the meeting, and approves resolutions with the absolute majority (maggioranza assoluta) of the overall votes relating to the shares held by the shareholders attending the meeting.
The following table summarizes the quorums required to (a) have the ordinary shareholders’ meeting validly held and (b) resolve upon the concerned matter.
|
Ordinary shareholders’ meeting |
|
|
Quorum necessary to validly hold the meeting |
Quorum to approve resolutions |
First call |
50%+1 of the overall votes relating to the shares issued by the Company |
50%+1 of the overall votes relating to the shares held by the shareholders who attend the meeting |
Second call |
N/A |
50%+1 of the overall votes relating to the shares held by the shareholders who attend the meeting |
118
Subsequent calls |
N/A |
50%+1 of the overall votes relating to the shares held by the shareholders who attend the meeting |
Single call |
N/A |
50%+1 of the overall votes relating to the shares held by the shareholders who attend the meeting |
Extraordinary Shareholders’ Meeting
The extraordinary shareholders’ meeting shall resolve upon amendments to the articles of association, the appointment, replacement and powers of the liquidators and other matters reserved to it by applicable law.
The extraordinary shareholders’ meeting is validly constituted and approve resolutions in first, second and any subsequent calls or, if so established in the Notice of Call, in a single call, with the quorums required by applicable law. For the purpose of calculating the quorums, the number of votes pertaining to the shares and not the number of shares is taken into account.
By virtue of the above, in first call, the extraordinary shareholders’ meeting is duly held with the presence of shareholders representing the majority of the overall votes relating to the shares issued by the Company, and approve resolutions with the favorable vote of at least two thirds of the overall votes relating to the shares held by the shareholders attending the meeting.
In second call, the extraordinary shareholders’ meeting is duly held with the presence of shareholders representing more than one third of the overall number of votes relating to the shares issued by the Company and approve resolutions upon with the favorable vote of at least two thirds of the overall votes relating to the shares held by the shareholders attending the meeting.
In subsequent calls, the extraordinary shareholders’ meeting is duly held with the presence of shareholders representing at least one fifth of the overall number of votes relating to the shares issued by the Company and approve resolutions with the favorable vote of at least two thirds of the overall votes relating to the shares held by the shareholders attending the meeting.
In a single call, the extraordinary shareholders’ meeting is duly held with the presence of shareholders representing at least one fifth of the overall number of votes relating to the shares issued by the Company and approve resolutions with the favorable vote of at least two thirds of the overall votes relating to the shares held by the shareholders attending the meeting.
The following table summarizes the majorities (quorum) required to (a) validly hold the ordinary shareholders’ meeting and (b) approve resolutions.
|
Extraordinary shareholders’ meeting |
|
|
Quorum necessary to validly hold the meeting |
Quorum to approve resolutions |
First call |
50%+1 of the overall votes relating to the shares issued by the Company |
Two thirds of the overall votes relating to the shares held by the shareholders who attend the meeting |
Second call |
More than one third of the overall votes relating to the shares issued by the Company |
Two thirds of the overall votes relating to the shares held by the shareholders who attend the meeting |
Subsequent calls |
One fifth of the overall votes relating to the shares issued by the Company |
Two thirds of the overall votes relating to the shares held by the shareholders who attend the meeting |
Single call |
One fifth of the overall votes relating to the shares issued by the Company |
Two thirds of the overall votes relating to the shares held by the shareholders who attend the meeting |
119
Right to Withdraw
Shareholders may exercise the right to withdraw from the Company in accordance with applicable law, with respect to all or part of their shareholding. Rights to withdraw are available to the shareholders who did not vote on or voted against resolutions relating to: (a) the extension of the term of the Company; or (b) the introduction or removal of limitations on share transfers.
For the purposes of the valid exercise of the right of withdrawal, the Beneficial Owners who exercise the right of withdrawal directly or through the Holder of Record, must prove that they were Beneficial Owners at the time of the adoption of the resolution from which the right of withdrawal arises and did not vote in favor of the such resolution.
The liquidation value of the shares is determined by reference to the arithmetic average of the closing prices during the six months preceding the publication of the Notice of Call for the meeting whose resolutions entitle the shareholders to withdraw.
Any agreement aimed at prohibiting or limiting the exercise of the right of withdrawal in the above cases would be null and void.
Corporate Governance of the Company
Pursuant to article 2409-sexiesdecies et seq. of the Italian Civil Code, the Company has adopted, a one-tier system of corporate governance (sistema monistico) according to which the management of the Company is carried out by the board of directors under the supervision of the Audit Committee (comitato per il controllo sulla gestione) set up within the board of directors.
Board of Directors
The board of directors shall be comprised of a number of members between a minimum of nine and a maximum of 15 members who shall remain in office for a term of no more than three financial years and may be re-elected for further terms. The office of the directors shall terminate on the date of the shareholders’ meeting convened to approve the financial statements for the third full financial year from their appointment (or such earlier date as may be determined by the shareholders). In addition, applicable law or the articles of association provide further causes of termination of a director’s appointment.
The directors must meet the eligibility and integrity requirements set out in article 2382 of the Italian Civil Code and have the professional qualifications required to perform their duties.
One third of the members of the board of directors, rounded up in case of fractional number, must meet the independence requirements set out in article 2399 of the Italian Civil Code.
The board of directors shall be entrusted with all powers for the ordinary and extraordinary management of the Company, with the authority to carry out all the acts deemed appropriate to achieve the corporate purpose, with the sole exception of those reserved for the shareholders’ meeting by law or the articles of association.
The board of directors is also responsible, pursuant to articles 2365, paragraph 2, and 2446, paragraph 3, of the Italian Civil Code, without prejudice to the concurrent competence of the extraordinary shareholders’ meeting, for resolutions concerning: (a) the merger and demerger of the Company in the cases provided for by articles 2505 and 2505-bis of the Italian Civil Code; (b) the transfer of the registered office within the Italian territory; (c) the establishment or closure of secondary offices; (d) the indication of the directors who have authority to represent the Company; (e) the reduction of the share capital in the event of withdrawal of a shareholder; (f) the reduction of the share capital following losses resulting in the Company’s own funds to be lower than two thirds of the share capital; and (g) the amendment of the articles of association necessary to reflect the enactment of laws or regulatory provisions or the conversion of the Company’s shares.
The board of directors shall elect a chairman from among its members, unless the shareholders’ meeting has already appointed one, and may also appoint one or more deputy chairmen.
120
The board of directors may also assign the office of “Honorary Chairman” to a person of recognized standing who has contributed to the growth and development of the Company. The office of Honorary Chairman may be granted to individuals who are not members of the board of directors, has an indefinite duration and can be revoked only for just cause. If he/she is not also a director, the Honorary Chairman may attend the meetings of the board of directors and the shareholders’ meeting only to express non-binding opinions on the issues to be resolved upon and may represent the Company only on the basis of special powers of attorney. The board of directors determines the remuneration, any other emolument and/or reimbursement of expenses due to the Honorary Chairman.
The board of directors may delegate part of its powers to an executive committee made up of some of its members or to one or more directors, determining their powers in compliance with the limitations set forth by applicable law. To this end, the provisions of article 2381, paragraphs 3, 4 and 5, of the Italian Civil Code shall apply.
The board of directors and, if appointed, the executive committee and the managing directors, within the limits of their powers, may appoint, among the Company’s employees, general managers or proxies, as well as, also among third parties, ad negotia or special proxies, determining their powers in compliance with the limitations set forth by applicable law.
The board of directors shall be convened, even outside the registered office, in Italy or abroad, every time the chairman deems it appropriate, or when it is requested by a managing director (if appointed) or by at least one third of its members. The meetings of the board of directors may also be held by audio or videoconference.
Even in the absence of a formal convocation, the board of directors shall be deemed to be duly held if all the directors in office are present.
In order for the resolutions of the board of directors to be valid, the presence of the majority of the directors in office and the favorable vote of the absolute majority of the directors attending are required.
Minutes of the meetings of the board of directors must be drawn up in accordance with applicable law, signed by the chairman of the meeting and by the secretary or notary, and must be copied in the book of meetings and resolutions of the board of directors.
With regard to resolutions concerning transactions in which one or more directors have an interest on their own behalf or on behalf of third parties, article 2391 of the Italian Civil Code shall apply.
Audit Committee
The Audit Committee shall be made up of three members, appointed by the board of directors, and its members shall remain in office for three financial years and may be re-elected. The Audit Committee shall elect a chairman from among its members.
The members of the Audit Committee shall meet the independence requirements set forth in article 2399 of the Italian Civil Code, and the additional independence requirements set forth in the relevant Italian and foreign laws and regulations applicable to the Company. Any member of the Audit Committee that is granted powers or holds particular offices for, or performs, even de facto, roles relating to, the management of the Company or of companies controlling it or controlled by it, shall thereupon automatically cease to be a member of the Audit Committee.
At least one member of the Audit Committee must be chosen among those enrolled in the Italian register of legal auditors, and shall possess the financial expertise required by the Italian and foreign laws and regulations applicable to the Company.
In case of death, resignation, revocation or disqualification of any members of the Audit Committee, or in case of loss by any members of the Audit Committee of the relevant independence and professional requirements, the board of directors shall promptly replace him or her by selecting new members among the other directors who meet such requirements.
The Audit Committee shall be entrusted with: (a) supervising the ongoing viability of the organizational structure of the Company, of the internal control system and of the administrative and accounting system, as well as its
121
suitability to properly represent the management facts; and (b) carrying out such further duties as entrusted to it by the board of directors, with particular regard to liaising with the firm appointed for the legal auditing of the accounts.
The Audit Committee shall also perform the duties pertaining to the Audit Committee pursuant to the provisions of U.S. laws and regulations applicable to the Company. The Audit Committee shall be convened at least every 90 days.
A meeting of the Audit Committee is duly held with the presence of the majority of its members and resolves by absolute majority of those present at the meeting. Any member who intends to disagree with the adoption of a resolution has the right to have the reasons for his disagreement recorded in the minutes.
The minutes of the meetings of the Audit Committee must be drawn up and signed by those present, and must be copied in the meeting book of the Audit Committee.
Election, Removal and Remuneration of Directors.
The board of directors is elected by the ordinary shareholders’ meeting according to a slate voting system. Directors remain in office for the period established by the shareholders meetings at the time of election, which cannot exceed three financial years, and may be re-elected.
According to the procedure provided for by the articles of association, the right to submit a slate for the election of the members of the board of directors is reserved to shareholders who hold, individually or jointly with other submitting shareholders, shares representing at least five per cent of the overall voting rights pertaining to the shares issued by the Company, it being understood that each shareholder, or group acting in concert, may submit only one slate. The ownership of the number of shares necessary for the presentation of the slate is determined based on the records of the Shareholders’ Register and the U.S. Register on the date on which the slates are deposited at the registered office, and according to the Record Date.
The slates shall: (i) be deposited at the Company’s registered office, pursuant to the Notice of Call, at least three days prior to the Record Date, and must be published by the Company in compliance with the applicable legal and regulatory provisions, if any; and (ii) indicate a number of proposed directors between 9 and 15, who shall meet the eligibility and integrity requirements provided by applicable law and the articles of association. Each slate must also indicate the candidate directors meeting the independence requirements set out in article 2399 of the Italian Civil Code (at least one third of the candidates), the candidate directors meeting the experience and independence requirements required by our articles of association (at least three candidates) and the candidate directors meeting the additional professional requirements required by the articles of association. Each proposed director shall only stand for election in one slate.
Each slate shall include: (a) the résumé of each of the proposed directors; (b) the statements by means of which each proposed director accepts his/her candidacy and states that he/she possesses the relevant eligibility, integrity, independence, expertise and professional requirements; and (c) the identity of the shareholders or of the Beneficial Ownership who have submitted the lists and of the percentage of voting rights attaching to the shares held by them.
Each shareholder can only vote for one slate of proposed directors, and such vote refers to the whole slate and, therefore, all the candidates indicated therein, without the possibility of variations, additions or exclusions.
The number of members of the board of directors shall be the same as the number of candidates indicated in the list that obtained the highest number of votes. The proposed directors indicated in the slate that obtained the highest number of votes shall be elected to the board of directors.
If more than one slate has obtained the same number of votes, a second ballot shall be held during the same shareholders’ meeting; only the slate obtaining the same number of votes shall take part in this second ballot.
In the event that, at the end of the voting, it is ascertained that one or more of the elected directors do not meet the relevant eligibility and integrity requirements, such candidates shall be excluded and, where necessary to ensure the correct composition of the board of directors, replaced in accordance with the following provisions.
122
In the event that, at the end of the voting, no directors are elected who meet the relevant independence, professional and expertise requirements, a number of candidates starting from the bottom of the slate must be excluded as is necessary to vacate the number of seats that are reserved to candidates who meet such requirements, to be appointed in accordance with the following provisions.
In the event that (a) no slates are submitted by the shareholders, (b) only one slate is submitted and this slate does not obtain the required majority of votes, (c) the number of elected directors is lower than nine, (d) only a number of directors, not the whole board, are to be appointed, or (e) it is not otherwise possible for any reason to appoint the board of directors following the above described procedure, the directors shall be appointed by the shareholders’ meeting without applying the slate voting mechanism, without prejudice to the obligation to ensure the correct composition of the board of directors and of the Audit Committee as required by law and the articles of association.
In the event that one or more directors cease to hold office during their term of office, the board of directors shall replace them with directors who meet the eligibility and integrity requirements and, where necessary to ensure the regular composition of the board of directors and of the Audit Committee, the independence, professional and expertise requirements provided by applicable law and the articles of association. To this end, the provisions of article 2386, paragraphs 1, 2 and 3, of the Italian Civil Code shall apply, without prejudice to the provisions of article 2409-octiesdecies, paragraph 4, of the Italian Civil Code and the provisions of the articles of association concerning the replacement of members of the Audit Committee.
If, following a director’s loss of the independence requirements and/or the independence and professional requirements set out in the articles of association, the board of directors and/or the Audit Committee are no longer compliant with the articles of association’s provisions, the director who no longer meets the aforementioned requirements must cease to be a director and be replaced.
The shareholders’ meeting establishes the compensation of the directors for their office as members of the board of directors, at the time of their appointment. The directors shall also be entitled to reimbursement of expenses incurred in the performance of their duties.
The shareholders’ meeting may also determine an aggregate amount for the compensation of all directors, including those holding specific functions, to be allocated by the board of directors.
The board of directors may provide for additional compensation for the directors entrusted with specific functions, which may consist of a fixed part and a variable part, correlated to the achievement of certain objectives, or consist of the right to subscribe for ordinary shares or other financial instruments of the Company at a predetermined price.
Furthermore, the shareholders’ meeting shall establish the fixed compensation of the chairman and the members of the Audit Committee for their entire term of office, at the time of their appointment. If the shareholders’ meeting does not do so, the compensation of the chairman and the members of the Audit Committee shall be established by the board of directors.
Liquidation.
The Company shall be wound up in the cases provided for by the Italian law.
In any case of winding-up of the Company, the extraordinary shareholders’ meeting shall determine the manner of liquidation and appoint one or more liquidators, determining their powers and remuneration, pursuant to article 2487 of the Italian Civil Code.
Shareholders agreements.
The shareholders’ agreements must be communicated to the Company and declared before each shareholders’ meeting. In case of failure to comply with these requirements, the voting rights attaching to the relevant shares cannot be exercised and any resolutions approved due to the favorable vote of such shares can be voided.
123
Material Differences in Italian law and our Articles of Association and Delaware Law
The provisions of the Italian Civil Code applicable to companies that are listed on a regulated market (società che fanno ricorso al mercato del capitale di rischio) differ from laws applicable to U.S. corporations and their shareholders. Set forth below is a summary of certain differences between the provisions of the Italian Civil Code applicable to companies that are listed on a regulated market (società che fanno ricorso al mercato del capitale di rischio) and the General Corporation Law of the State of Delaware relating to shareholders’ rights and protections. This summary is not intended to be a complete discussion of the respective rights and it is qualified in its entirety by reference to the laws of the Republic of Italy and of the State of Delaware.
Items |
Republic of Italy |
State of Delaware |
Number of Directors |
Under Italian law, the board of directors is appointed by the ordinary shareholders’ meeting of the corporation, for the period established at the time of appointment, which cannot exceed three financial years.
The number of directors is determined by the articles of association or, if only a minimum and a maximum number of directors is provided, by the shareholders’ meeting.
For corporations adopting the one-tier board system, the board of directors appoints among its members the audit committee which is composed of at least 3 directors.
The board of directors appoints the chairman among its members, if not appointed by the shareholders’ meeting.
A director may be reappointed for successive terms. |
Under Delaware law, a corporation must have at least one director and the number of directors shall be fixed by or in the manner provided in the By-Laws unless the certificate of incorporation fixes the number of directors, in which case a change in the number shall be made only by amendment of the certificate of incorporation. |
Removal of Directors |
Under Italian law, directors may be removed from office at any time by the shareholders’ meeting. A director that is removed without cause may have a claim for damages against the corporation. Directors may resign at any time by written notice to the board of directors and to the chairman of the audit committee. |
Under Delaware law, any director or the entire board of directors may be removed, with or without cause, by the holders of a majority of the shares entitled to vote at an election of directors, except (i) unless the certificate of incorporation provides otherwise, in the case of a corporation whose board of directors is classified, shareholders may effect such removal only for cause, or (ii) in the case of a corporation having cumulative voting, if less than the entire board of directors is to be removed, no |
124
|
|
director may be removed without cause if the votes cast against his removal would be sufficient to elect him if then cumulatively voted at an election of the entire board of directors, or, if there are classes of directors, at an election of the class of directors of which he/she is a part. |
Vacancies on the Board of Directors |
Under Italian law, vacancies arising from resignation, removal, death, or loss of the required legal capabilities or independence requirements of directors shall be filled by a resolution of the board of directors, with the approval of the audit committee. The newly appointed directors shall serve until the next ordinary shareholders’ meeting, by which they may be confirmed or substituted. In case of resignation, removal, death, or loss of the required legal capabilities or of the independence requirements of/by more than half of the directors originally appointed by the shareholders’ meeting, the remaining directors must call an ordinary shareholders’ meeting promptly to appoint as many directors as necessary to fill the vacancies and until such vacancies are so filled the resigning directors, if any, remain temporarily in office (“in prorogatio”). |
Under Delaware law, vacancies and newly created directorships may be filled by a majority of the directors then in office (even though less than a quorum) or by a sole remaining director unless (i) otherwise provided in the certificate of incorporation or By-Laws of the corporation or (ii) the certificate of incorporation directs that a particular class of stock is to elect such director, in which case a majority of the other directors elected by such class, or a sole remaining director elected by such class, will fill such vacancy. |
Annual General Meeting |
Under Italian law, shareholders’ meetings can be either ordinary or extraordinary.
The ordinary shareholders’ meeting of corporations adopting the one-tier board system, inter alia, o approves the corporation’s financial statements; o appoints and removes the directors; o appoints external auditors; o determines the basic compensation of directors and of external auditors; |
Under Delaware law, the annual meeting of stockholders shall be held at such place, on such date and at such time as may be designated from time to time by the board of directors or as provided in the certificate of incorporation or by the By-Laws. |
125
|
o resolves on the initiation of a liability action against the company’s directors o resolves on the authorizations, if any, required by the articles of association for carrying out certain transactions. |
|
|
Ordinary shareholders’ meeting must be convened at least once a year within the term established by the articles of association and in any case not later than 120 days after the end of the financial year. |
|
|
Such term may be extended to up to 180 days after the end of the financial year, if the corporation is bound by law to draw up consolidated financial statements or if particular circumstances concerning its structure or its purposes so require. |
|
Special Meeting |
Under Italian law the special shareholders’ meeting (also referred to as “extraordinary meeting”), inter alia, (i) resolves on amendments to the articles of association; (ii) appoints, replaces and sets forth the powers of liquidators; and (iii) resolves on any other matter assigned to it by law. |
Under Delaware law, special meetings of the stockholders may be called by the board of directors or by such person or persons as may be authorized by the certificate of incorporation or by the By-Laws. |
Location of the General Meeting |
Shareholders’ meetings may be held in the municipality where the corporation has its registered office or in the locations determined by the board of directors in compliance with the provisions of the articles of association. If so permitted by the articles of association shareholders’ meeting may be also held via teleconference. |
Shareholder meetings may be held within or outside the State of Delaware and may be held virtually if so permitted in accordance with the certificate of incorporation or the By-Laws. |
Action by Written Consent |
Actions required under Italian law to be taken by a meeting of shareholders may not be taken by the shareholders without a meeting. |
Any action required to be taken by a meeting of shareholders may be taken without a meeting if a consent for such action is in writing and is signed by shareholders having not less than the minimum number of votes that would be necessary to authorize or take such action at a |
126
|
|
meeting at which all shares entitled to vote thereon were present and voted. |
Notice of General Meeting |
Under Italian law, a shareholders’ meeting is convened by the board of directors by means of a written notice containing the date, time and place of the meeting and a list of the items on the agenda.
The notice must be published in the Official Gazette of the Italian Republic or in at least one daily newspaper indicated in the articles of incorporation at least 15 days before the date set for the meeting. The articles of association may also provide for additional requirements, such as the publication of the notice on the website of the corporation.
Unless otherwise provided by the articles of association, the notice of a shareholders’ meeting may specify two or more meeting dates for an ordinary or extraordinary shareholders’ meeting. |
Under Delaware law, unless otherwise provided in the certificate of incorporation or By-Laws, written notice of any meeting of the stockholders must be given to each stockholder entitled to vote at the meeting not less than 10 and no more than 60 days before the date of the meeting and shall specify the place, date, hour, and purpose or purposes of the meeting. |
Quorum |
Unless otherwise provided for by the articles of association, ordinary shareholders’ meetings are validly held, in a single call, irrespective of the percentage of the voting share capital present at the meeting and resolutions are validly passed with the majority (i.e., 50%+1) of the voting share capital present at the meeting.
The Company’s articles of association may provide that ordinary shareholders’ meetings are held in multiple calls. In such case, unless higher majorities are provided for by the articles of association with regard to certain resolutions, (i) on first call, ordinary shareholders’ meetings are validly held if the majority of the voting share capital is present at the meeting and resolutions are validly passed with the majority (i.e., 50%+1) of the voting share capital present at the meeting, and (ii) on second call, and in any subsequent |
The certificate of incorporation or By-Laws may specify the number of shares, the holders of which shall be present or represented by proxy at any meeting in order to constitute a quorum, but in no event shall a quorum consist of less than one third of the shares entitled to vote at the meeting. In the absence of such specification in the certificate of incorporation or By-Laws, a majority of the shares entitled to vote, present in person or represented by proxy, shall constitute a quorum at a meeting of stockholders. |
127
|
calls, are validly held irrespective of the percentage of the voting share capital present at the meeting and resolutions are validly passed with the majority (i.e., 50%+1) of the voting share capital present at the meeting. |
|
|
Unless otherwise provided by the company’s articles of association, extraordinary shareholders’ meetings are validly held, in a single call, if shareholders representing at least one fifth of the voting share capital is present at the meeting and resolutions are validly passed with the favorable vote of at least two thirds of the voting share capital present at the meeting. |
|
|
The articles of association may provide that extraordinary shareholders’ meetings are held in multiple calls. In such case, unless higher majorities are provided by the articles of association, (i) on first call, extraordinary shareholders’ meetings are validly held if the majority (i.e., 50%+1) of the voting share capital is present at the meeting and resolutions are validly passed with the favorable vote of at least two thirds of the voting share capital present at the meeting; (ii) on second call, extraordinary shareholders’ meetings are validly held if more than one third of the voting share capital is present at the meeting and resolutions are validly passed with the favorable vote of at least two thirds of the voting share capital present at the meeting, (iii) in subsequent calls, extraordinary shareholders’ meetings are validly held if shareholders representing at least one fifth of the voting share capital is present at the meeting and resolutions are validly passed with the favorable vote of at least two thirds of the voting share capital present at the meeting. |
|
Proxy |
A shareholder may designate another person to attend, speak and vote at the shareholders’ meetings of |
Under Delaware law, at any meeting of stockholders, a stockholder may designate another person to act for |
128
|
the corporation on such shareholder’s behalf by way of a written proxy. By means of each proxy, a shareholder may confer to the relevant attorneys the power to attend, speak and vote to a single shareholders’ meeting. The proxy must include the names of the attorneys and of the substitutes, if any. A shareholder may not appoint as proxy-holder directors or employees of the corporation or of companies controlled by the latter. |
such stockholder by proxy, but no such proxy shall be voted or acted upon after three years from its date, unless the proxy provides for a longer period. A director of a Delaware corporation may not issue a proxy representing the director’s voting rights as a director. |
|
A single proxy-holder may not hold power of attorney for a maximum number of shareholders comprised between 20 and 200, depending on the amount of the company’s corporate capital. |
|
|
A director may not issue a proxy to confer to another person his/her voting rights as a director. |
|
Preemptive Rights |
Pursuant to Italian law, shareholders are entitled to subscribe for newly issued shares in proportion to their respective shareholdings. Subject to certain conditions, such pre-emptive rights may be waived or limited by the articles of associations (up to 10 percent of the existing corporate capital) or by a resolution of the extraordinary shareholders’ meeting. |
Under Delaware law, shareholders have no preemptive rights to subscribe to additional issues of stock or to any security convertible into such stock unless, and except to the extent that, such rights are expressly provided for in the certificate of incorporation. |
|
In such event, the proposal concerning the issuance of new shares must be justified by the board of directors and the relevant subscription price must be determined based on the value of the consolidated net worth of the corporation. External auditors of the corporation must issue an opinion on the fairness of the newly issued shares’ subscription price. |
|
|
Pre-emptive rights may also be limited with respect to newly issued shares when these are offered for subscription by employees of the |
|
129
|
corporation or its subsidiaries or parent companies. |
|
Authority to Allot |
The extraordinary shareholders’ meeting may increase the share capital and issue new shares (i) to be subscribed by the current shareholders or third parties for a consideration or (ii) by allotting the newly issued shares to the current shareholders for no consideration, provided, in such latter case, that there are sufficient available reserves to cover such newly issued shares, the share capital is covered by the existing own funds of the corporation. The extraordinary shareholders’ meeting may delegate the power to increase the share capital of the corporation and/or issue new shares to the board of directors up to a specified amount and for a maximum period of 5 years since the date of such delegation. |
Under Delaware law, if the corporation’s charter or certificate of incorporation so provides, the board of directors has the power to authorize the issuance of stock. It may authorize capital stock to be issued for consideration consisting of cash, any tangible or intangible property or any benefit to the corporation or any combination thereof. It may determine the amount of such consideration by approving a formula. In the absence of actual fraud in the transaction, the judgment of the directors as to the value of such consideration is conclusive. |
|
In case new shares are issued for cash consideration, the relevant resolution may be executed upon subscription of the new shares and payment of at least 25 per cent of their nominal value and the entire share premium by the subscribers. |
|
Liability of Directors and Officers |
Directors of the corporation may be held liable towards the corporation, the creditors of the corporation or single shareholders or creditors for any damage caused to them in consequence of a breach of the directors’ general or specific duties and obligations.
Any provision, whether contained in the corporation’s articles of association or any contract or otherwise, that purports to exempt directors in connection with breach of duty in relation to the corporation may not be enforceable.
Apart from insolvency or special circumstances, a judicial action for damages may be brought against the |
Under Delaware law, a corporation’s certificate of incorporation may include a provision eliminating or limiting the personal liability of a director to the corporation and its stockholders for damages arising from a breach of fiduciary duty as a director. However, no provision can limit the liability of a director for:
• any breach of the director’s duty of loyalty to the corporation or its stockholders; • acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; • intentional or negligent payment of unlawful dividends |
130
|
directors only by the corporation (upon resolution of an ordinary shareholders’ meeting), one or more shareholders owning at least 2.5 per cent of the share capital, or by single shareholders or creditors (only in case of damages directly suffered by the latter), as the case may be. |
or stock purchases or redemptions; or • any transaction from which the director derives an improper personal benefit.
|
|
The corporation may waive or settle actual or potential claims against directors, provided that one or more shareholders owning at least 5 per cent of the share capital do not object to the waiver or settlement. |
|
Voting Rights |
Generally, each shareholder is entitled to one vote for each share held by such shareholder at all shareholders’ meetings of the corporation. |
Delaware law provides that, unless otherwise provided in the certificate of incorporation, each stockholder is entitled to one vote for each share of capital stock held by such stockholder. |
|
The articles of association may provide that certain share classes carry no, limited, contingent or multiple (up to 3 votes per share) voting rights. |
|
Shareholder Vote on Certain Transactions |
Resolutions approving any merger or demerger of the corporation require the approval of the board of directors and the approval of the extraordinary shareholders’ meeting of the corporation (please refer to paragraph “Quorum” above for further details). The articles of association may provide for other transactions to be subject to the authorization of the ordinary shareholders’ meeting of the corporation. In such event, unless otherwise provided by the articles of association, the relevant transaction must be approved with the favorable vote of the ordinary shareholders’ meeting (please refer to paragraph “Quorum” above for further details). |
Generally, under Delaware law, unless the certificate of incorporation provides for the vote of a larger portion of the stock, completion of a merger, consolidation, sale, lease or exchange of all or substantially all of a corporation’s assets or dissolution requires: • the approval of the board of directors; and • approval by the vote of the holders of a majority of the outstanding stock or, if the certificate of incorporation provides for more or less than one vote per share, a majority of the votes of the outstanding stock of a corporation entitled to vote on the matter. |
Standard of Conduct for Directors |
Directors have a general duty to act with care, without self-interest and on a well- informed basis. |
Delaware law does not contain specific provisions setting forth the standard of conduct of a director. |
131
|
The applicable standard of conduct is determined, on a case-by-case basis, taking into account the characteristics of the corporation, the specific tasks and responsibilities conferred to the single directors, and the personal skills of the latter.
In addition, directors have numerous specific duties and obligations, such as, inter alia:
• keeping the corporation’s books, records and other databases (including the shareholders’ register) in such a manner that the corporation’s rights and obligations may be ascertained from the interested parties at all times; • preparing the corporation’s annual financial statements according with the applicable accounting principles and filing them with the Companies’ Register on time; • registering the corporation with the Companies’ Register and keeping the registered information up to date; • convening annually or when necessary or required by one or more shareholders holding at least 5 per cent of the corporate capital, the ordinary shareholders’ meetings; and • monitoring the own funds and financial position of the corporation and initiate the actions or procedures contemplated by the law in case of (i) losses entailing the reduction of the share capital of the corporation below the threshold of two thirds of the share capital or (ii) income, asset or financial unbalances having certain characteristics. |
The scope of the fiduciary duties of directors is generally determined by the courts of the State of Delaware. In general, directors have a duty to act without self-interest, on a well- informed basis and in a manner they reasonably believe to be in the best interest of the stockholders. Directors of a Delaware corporation owe fiduciary duties of care and loyalty to the corporation and to its shareholders. The duty of care generally requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself of all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he/ she reasonably believes to be in the best interests of the corporation. He/she must not use his corporate position for personal gain or advantage. In general, but subject to certain exceptions, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties. Delaware courts have also imposed a heightened standard of conduct upon directors of a Delaware corporation who take any action designed to defeat a threatened change in control of the corporation. |
|
|
In addition, under Delaware law, when the board of directors of a |
132
|
|
Delaware corporation approves the sale or break-up of a corporation, the board of directors may, in certain circumstances, have a duty to obtain the highest value reasonably available to the shareholders. |
Indemnification of Directors and Officers |
Corporations may enter into indemnity agreements (patti di manleva) with directors, according to which the latter are kept harmless from the liabilities arising from the acts they carried out during their office. Further, when directors resign from their office, corporations may issue indemnification letters in their favor. |
A corporation may indemnify a director or officer of the corporation against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in defense of an action, suit or proceeding by reason of such position if (i) such director or officer acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation and (ii) with respect to any criminal action or proceeding, such director or officer had no reasonable cause to believe his conduct was unlawful. |
Shareholder Litigation |
Under Italian law, liability actions against directors may be brought by the corporation following a resolution of the ordinary shareholders’ meeting.
The statute of limitation for this action is equal to five years from the termination of the relevant director’s appointment.
The approval of the liability action by the shareholders’ meeting implies the removal from office of the director against whom it is proposed—if the latter is still in office—provided that it is taken with the favorable vote of at least 20 per cent of the share capital. |
Under Delaware law, a stockholder may initiate a derivative action to enforce a right of a corporation if the corporation fails to enforce the right itself. The complaint must: • state that the plaintiff was a stockholder at the time of the transaction of which the plaintiff complains or that the plaintiffs shares thereafter devolved on the plaintiff by operation of law; and • allege with particularity the efforts made by the plaintiff to obtain the action the plaintiff desires from the directors and the reasons for the plaintiff’s failure to obtain the action; or • state the reasons for not making the effort. |
|
The corporation may waive the exercise of the liability action and may settle, provided that the waiver and the settlement are approved by a resolution of the ordinary shareholders’ meeting, and provided |
Additionally, the plaintiff must remain a stockholder through the duration of the derivative suit. The action will not be dismissed or compromised without the approval of the Delaware Court of Chancery. |
133
|
that there is no vote against by a minority of shareholders representing at least 5 per cent of the share capital. |
|
|
Liability actions may also be brought by shareholders holding at least 2.5 per cent of the share capital or the lower amount set forth in the corporation’s articles of association. |
|
|
The shareholders who have acted may waive or settle the action; any consideration for the waiver or settlement shall inure to the benefit of the corporation. |
|
Amendment of the Certificate of Incorporation |
Certificate of incorporation is not a separate document from the articles of association and, as such, is not separately amended. |
Under Delaware law, generally a corporation may amend its certificate of incorporation if: • its board of directors has adopted a resolution setting forth the amendment proposed and declared its advisability; and • the amendment is adopted by the affirmative votes of a majority (or greater percentage as may be specified by the corporation) of the outstanding shares entitled to vote on the amendment and a majority (or greater percentage as may be specified by the corporation) of the outstanding shares of each class or series of stock, if any, entitled to vote on the amendment as a class or series. |
Amendment of By-Laws /Articles of Association |
Under Italian law, the extraordinary shareholders’ meeting must resolve upon any amendments to the corporation’s articles of association, which amendments must also be filed with the Companies’ Register. The articles of association may provide for the board of directors’ power to carry out other amendments to the corporation’s articles of association, as to, inter alia, resolutions regarding the setting-up or closure of the corporation’s branch office, |
Under Delaware law, the stockholders entitled to vote have the power to adopt, amend or repeal By-Laws. A corporation may also confer, in its certificate of incorporation, that power upon the board of directors. |
134
|
simplified mergers (e.g., a merger in which the merging corporation owns all or at least 90% of the share capital of the merged corporation), the indication of whom among the directors has the power to represent the corporation. |
|
|
Upon each of the amendments to the corporation’s articles of association, the up-to-date version must be filed with the Companies’ Register. |
|
Transactions with Significant Shareholders |
Relevant rules are not applicable under Italian law for companies whose shares are not listed on a regulated market in the EU. |
Subject to certain exceptions and conditions, a corporation may not enter into a business combination with an interested shareholder for a period of three years from the time the person became an interested shareholder without prior approval from shareholders holding at least 66 2/3% of the corporation’s outstanding voting stock which is not owned by such interested shareholder. |
Dissenters’ Rights of Appraisal |
Mergers and demergers’ plans to be approved by the board of directors must be based on a fair shares’ exchange ratio, to be certified by independent experts, appointed by the court. Such experts must draw up a report on the fairness of the exchange ratio of the shares, stating: a) the method or methods followed in determining the proposed exchange ratio and the values resulting from the application of each such method or methods; b) any valuation difficulties; and c) an opinion on the appropriateness of the methods followed to determine the exchange ratio and the relevant importance attributed to each in determining the value adopted. |
Appraisal rights shall be available for the shares of any class or series of stock of a corporation in a merger or consolidation, subject to limited exceptions, such as a merger or consolidation of corporations listed on a national securities exchange in which listed stock is the offered consideration. |
C. Material Contracts
Please see the section entitled “Item 4. Information on the Company—B. Business Overview” for more information concerning our material contracts.
D. Exchange Controls
There are no governmental laws, decrees or regulations in Italy generally restricting the import or export of capital or affecting the remittance of dividends, interest or other payments to non-resident holders of ordinary shares. There
135
are no limitations under Italian law or the company’s articles of association on the right of non-resident or foreign owners to be registered holders of, or to exercise voting rights in relation to, ordinary shares.
E. Taxation
The following summary of Italian and U.S. federal income tax considerations of an investment in our ordinary shares is based upon laws and relevant interpretations thereof in effect as of the date of this annual report, all of which are subject to change. This summary does not deal with all possible tax considerations relating to an investment in our ordinary shares, such as the tax considerations under U.S. state and local tax laws or under the tax laws of jurisdictions other than Italy and the United States.
Italian Taxation
This section describes solely the material Italian tax consequences of acquiring, holding and disposing of the Shares. It does not consider every aspect of Italian taxation that may be relevant to a particular holder of Shares in special circumstances or who is subject to special treatment under applicable law, and it is not intended to be applicable in all respects to all classes of investors.
Shareholders and any potential prospective investors should consult their own tax advisors regarding the Italian tax consequences of acquiring, holding and disposing of Shares in their particular circumstances and should investigate the nature and the origin of the amounts received as distributions in connection with the Shares (dividends or reserves).
Where in this section English terms and expressions are used to refer to Italian concepts, the meaning to be given to these terms and expressions shall be the meaning to be given to the equivalent Italian concepts under Italian tax law. This summary assumes that Shares will be listed on a regulated market as defined under the interpretation of the Italian tax authorities. This summary also assumes that Stevanato is organized, and that the business will be conducted, in the manner outlined in this annual report. A change to the organizational structure or to the manner in which Stevanato conducts its business may invalidate the contents of this section, which will not be updated to reflect any such change.
This summary is based on the tax laws of the Republic of Italy and published case law / practice (unpublished case law / practice is not included) as it stands at the date of this annual report. The law upon which this description is based is subject to change, potentially with retroactive effect. Any such change may invalidate the contents of this description, which will not be updated to reflect this change.
Definitions
For purposes of this section of this annual report, the terms defined have the meaning described below.
References to “CITA” are to Presidential Decree No. 917 of December 22, 1986 (the Consolidated Income Tax Act).
References to “Italian White List” are to the list of countries and territories allowing a satisfactory exchange of information with Italy (i) currently included in the Italian Ministerial Decree of September 4, 1996, as subsequently amended and supplemented or (ii) once effective in any other decree or regulation that will be issued in the future to provide the list of such countries and territories (and that will replace Ministerial Decree of September 4, 1996), including any country or territory that will be deemed listed therein for the purpose of any interim rule.
References to “Non-Qualified Shareholdings” are to shareholdings in companies listed on regulated markets other than Qualified Shareholdings;
References to “Qualified Shareholdings” are to shareholdings in companies listed on regulated markets represented by the ownership of shares (other than savings shares), rights or securities through which shares may be acquired which represent overall voting rights exercisable at ordinary shareholders’ meetings of over 2 percent or an interest in the share capital of over 5 percent;
136
References to “Transfer of Non-Qualified Shareholdings” are to transfers of shares (other than savings shares), rights or securities through which shares can be acquired, different from the Transfer of Qualified Shareholdings; and
References to “Transfer of Qualified Shareholdings” are to transfers of shares (other than savings shares), rights or securities through which shares can be acquired, which exceed, over a period of twelve months, the threshold for their qualification as Qualified Shareholdings. The 12-month period starts from the date on which the securities and the rights owned represent a percentage of voting rights or interest in the capital exceeding the aforesaid threshold. For rights or securities through which holdings can be acquired, it is considered the percentage of voting rights or interest in the capital potentially attributable to the holdings.
Tax Regime for Dividends
Dividends allocated to the Shares will be subject to the tax treatment ordinarily applicable to dividends paid by joint stock companies resident in Italy for tax purposes.
The following different methods of taxation are provided for the different classes of recipients.
Dividends received by individual shareholders who are resident in Italy for income tax purposes in connection with a Non-Qualified Shareholding, not holding the participation in connection with a business activity, are subject to a final withholding tax (“WHT”) at the rate of 26 percent pursuant to the article 27 of Presidential Decree No. 600 of September 29, 1973 (hereinafter “Decree 600/73”)—which will be withheld by Stevanato upon payment of the dividend—and do not have to be reported in the shareholders’ annual income tax return.
Dividends paid to individual shareholders who have entrusted the management of their financial assets, including the Shares, to an authorized intermediary and have expressly elected for the discretionary investment portfolio regime (Regime del Risparmio Gestito, set forth by article 7 of Legislative Decree No. 461 of November 21, 1997 (“Decree 461/97”), as illustrated below) are not subject to WHT, and are included in the computation of the accrued annual increase in value of the managed assets, subject to an ad hoc 26 percent substitute tax withheld by the authorized intermediary pursuant to article 7(4) of Legislative Decree No. 461 of November 21, 1997.
Dividends received by resident individual shareholders, holding the Shares in connection with a business activity, are not subject to WHT, if the individual shareholders declare to the payor before the payment of the dividends that the profits collected are from holdings related to the business activity. Such dividends are partially included in the individual shareholders’ taxable income, subject to personal income tax (“IRPEF”) for (i) 58.14 percent of their amount as to dividends paid out of profits realized in the tax years following the one in progress on December 31, 2016, (ii) 49.72 percent of their amount as to dividends paid out of profits realized from the tax year following the one in progress on December 31, 2007 up to the one in progress on December 31, 2016 and (iii) 40 percent of their amounts as to dividends paid out of profits realized in the tax years up to that in progress on December 31, 2007. For these purposes (taxation of the recipient), profits realized in the tax years up to the tax year in progress on December 31, 2007, and then profits realized in the tax years up to the tax year in progress on December 31, 2016 are deemed to be distributed with priority. IRPEF is generally levied at progressive rates ranging from 23 percent to 43 percent, plus local surcharges.
Dividends received by resident individual shareholders not engaged in a business activity, in connection with a Qualified Shareholding not held in the context of the discretionary investment portfolio regime, are subject to the same 26 percent WHT applicable in connection with dividends received on Non-Qualified Shareholding and do not have to be reported in the shareholders’ annual income tax return. However, with respect to dividends paid on a Qualified Shareholding out of profits realized in the tax years up to that in progress on December 31, 2017, the previously applicable regime would continue to apply, provided that the distribution of such profits is approved between January 1, 2018 and December 31, 2022. Hence, such dividends would not be subject to any WHT and would be included in the individual shareholder’s taxable income according to the rules illustrated above for individual shareholders holding the Shares in connection with a business activity.
137
Dividends received by partnerships (other than non-commercial partnership) and similar entities as referred to in article 5 of the CITA, as well as to companies or entities as referred to in article 73 (1) sections a) and b) of the CITA, such as joint stock companies, partnerships limited by shares, limited liability companies, public and private entities (other than companies) and trusts whose sole or principal purpose is to carry on a business activity, which are resident in Italy for income tax purposes, are not subject to WHT at source and are included in the recipient’s overall taxable income.
In particular, dividends received by:
For some types of businesses and under certain conditions, the dividends received will also be included for 50 percent of their amounts in the taxable income subject to the Regional Tax on Business Activities (“IRAP”).
Dividends received by non-commercial entities which are resident in Italy for income tax purposes are not subject to WHT and are included in the recipient’s overall taxable income for 50 percent of their amount subject to IRES. However, non-commercial entities should account for a non-distributable reserve equal to IRES that would have been paid had the exempt portion of dividend been subject to tax.
Dividends received by Italian residents exempt from IRES are generally subject to WHT at a rate of 26 percent. No Italian tax is levied at source on Italian entities that are excluded from income taxation pursuant to article 74(1) of the CITA.
Dividends received by Italian pension funds established pursuant to article 17 of Legislative Decree No. 252 of December 5, 2005 are not subject to WHT and are included in the annual net accrued results of the pension fund, which is subject to a substitute tax of 20 percent. Subject to certain limitations and requirements (including a minimum holding period), dividends received by certain pension funds, not in connection with a Qualified Shareholding, may be exempt from any taxation if the shares meet the requirements set by to article 1(88-114) of Law No. 132 of December 11, 2016 (“Italian Budget Law for 2017”).
Dividends received by Italian undertakings for collective investment of saving income (OICR) and Luxembourg based OICR which have already been authorized for sale in Italy, subject to supervision, other than real estate
138
investment funds and by Italian investment companies with variable or fixed capital (SICAV and SICAF), are not subject to WHT. Dividends received by the aforementioned investment funds are not subject to tax at the level of such entities pursuant to article 73(5-quinquies) of the CITA.
They are generally subject to taxation upon the investor at the time of payment or when the units of the aforementioned investment funds are transferred or redeemed.
Dividends received by Italian-resident real estate investment funds established pursuant to article 37 of Legislative Decree No. 58 of 1998, and article 14-bis of Law No. 86 of January 25, 1994, and by Italian real estate SICAF are not subject to WHT pursuant to Law Decree No. 351 of September 25, 2001.
In some circumstances, the income realized by an Italian non-institutional real estate investment funds may be attributed to their non-institutional investors (thus being included in their income taxable in Italy) holding an investment of more than 5 percent of the fund assets.
No Italian WHT at source is levied on dividends paid to non-resident persons that hold the Shares through a permanent establishment in Italy to which the Shares are effectively connected. Only 5 percent of the dividends are included in the overall income subject to IRES, unless the Shares are booked as shares held for trading by holders applying the International Financial Reporting Standards as issued by the International Accounting Standards Board. In this case, dividends would be fully included in the recipient’s taxable income for IRES purposes.
For some types of businesses and under certain conditions, dividends are also included in the net value of production, which is subject to IRAP.
A WHT at a rate of 26 percent is generally levied on dividends paid to non-resident persons that do not have a permanent establishment in Italy to which the Shares are effectively connected.
Subject to a specific application that must be submitted to the Italian tax authorities under the terms and conditions provided by law, non-resident holders are entitled to a tax relief (in the form of a refund), which cannot be greater than 11/26 (eleven twenty-sixths) of the tax levied in Italy, if they can demonstrate that they have paid final tax abroad on the same profits.
As an alternative to the tax relief described above, persons resident in Countries that have a double tax treaty in force with Italy may request that the WHT on dividends be levied at the (reduced) rate provided under the applicable double tax treaty. Under article 10 of the Italy-U.S. double tax treaty (a) treaty entitled U.S. resident shareholders can generally benefit from a reduced WHT rate on dividends equal to 15 percent, (b) treaty entitled U.S. resident companies can benefit, under certain conditions, from a reduced WHT rate on dividends equal to 5 percent, and (c) certain qualified U.S. governmental entities are entitled, under certain conditions, to a full exemption from WHT on dividends.
The domestic WHT rate on dividends is 1.2 percent (and not 26 percent) if the recipients and beneficial owners of the dividends are companies or entities that are (a) resident for tax purposes in an EU Member State or in a State that is party to the European Economic Area Agreement (“EEA Member State”) and is included in the Italian White List and (b) subject to corporate income tax in such State. These companies and entities are not entitled to the tax relief described above.
The domestic WHT rate on dividends is 11 percent (and not 26 percent) if the recipients and beneficial owners of the dividends are pension funds that are set up in an EU Member States or an EEA Member State included in the Italian White List. These pension funds are not entitled to the tax relief described above.
Moreover, article 1(631-632) of Law No. 178 of December 30, 2020 (“2021 Budget Law”) has introduced favorable tax regime applicable to certain for undertakings for collective investment (“UCIs”) established outside of
139
Italy according to which dividends derived from shareholdings in Italian tax resident companies are not subject to taxation in Italy, if realized by: (i) foreign UCIs compliant with Directive 2009/65/EC (UCITS Directive), or (ii) foreign UCIs (not compliant with Directive 2009/65/EC) established in an EU Member State or EEA Member State allowing for an adequate exchange of information for tax purposes and whose manager is subject to regulatory supervision in the Country where it is established pursuant to Directive 2011/61/EU (AIFM Directive).
Under article 27-bis of Decree 600/73, which implemented in Italy Directive 435/90/EEC of July 23, 1990, then recast in EU Directive 2011/96 of November 30, 2011 (the “Parent-Subsidiary Directive”), a company is entitled to a full refund of the WHT levied on the dividends if it (a) has one of the legal forms provided for in the appendix to the Parent-Subsidiary Directive, (b) is resident for tax purposes in an EU Member State without being considered to be resident outside the EU according to a double tax treaty signed with a non-EU country, (b) is subject in the Country of residence to one of the taxes indicated in the appendix to the Parent-Subsidiary Directive with no possibility of benefiting from optional or exemption regimes that have no territorial or time limitations and (d) directly holds Shares that represent an interest in the issued and outstanding capital of Stevanato of no less than 10 percent for an uninterrupted period of at least one year. If these conditions are met, and as an alternative to submitting a refund request after the dividend distribution, the nonresident company may request that no tax be levied at the time the dividends are paid, provided that (x) the 1-year holding period under condition (d) above has already run and (y) the non-resident company promptly submits proper documentation. EU resident companies that are controlled directly or indirectly by persons that are not resident in a EU Member State may request the refund or the direct withholding exemption only if the EU resident companies prove that they do not hold the Shares for the sole or primary purpose of benefiting from the Parent-Subsidiary Directive.
The application of the above-described tax relief, WHT reduction under the double tax treaties or WHT exemption, is subject to conditions required under the applicable laws and/or treaties, which may vary depending on the case, as well as to the fulfillment by the shareholders of certain formalities, such as the timely provision to the withholding tax agent of affidavits, self-statements and tax residence certificates. In this respect, shareholders should consult with their own independent tax advisors to determine whether they are eligible for, and how to obtain, such tax relief, WHT reductions or exemption.
Distributions of Certain Capital Reserves
Special rules apply to the distribution of certain capital reserves, including reserves or funds created with share offerings’ premiums, adjusted interest paid by subscribers of shares, capital contributions, capital account payments made by shareholders or tax-exempt monetary revaluation funds. Under certain circumstances, such distribution may trigger taxable income in the hands of the recipients depending on the existence of current profits or outstanding profit reserves of the distributing company at the time of the distribution, and on the actual nature of the reserves so distributed. The application of such rules may also have an impact on the tax basis of the shares and the characterization of the taxable income received by the recipients as well as the tax regime applicable to it. Non-Italian resident shareholders may be subject to tax in Italy as a result of the distribution of such reserves pursuant to the same tax regime applicable to dividends as described at section “Tax Regime for Dividends” above. Prospective investors should consult their advisers in case any distributions of such capital reserves occur.
Tax Regime for Capital Gains Realized Upon Transfer of Shares
Capital gains, other than those realized in connection with the carrying out of a business activity, realized by individuals resident in Italy for tax purposes upon transfer for consideration of shares are subject to the same tax regime whether they are realized upon Transfer of Qualified Shareholdings or Transfer of Non-Qualified Shareholdings.
In particular, such capital gains are subject to substitute tax at a rate of 26 percent. The taxpayer may opt for one of the following three regimes:
140
Capital gains realized by partnerships and similar entities or Italian residents on the sale or disposal of the Shares held in connection with a business activity, are included in the recipients’ overall taxable income for the entire amount in the tax year in which they are realized, subject to income tax at ordinary rates. However, if the conditions indicated in the following paragraph for the partial exemption provided for capital gains realized by Italian resident companies and commercial entities were satisfied, these capital gains would be subject to tax only partially, in an amount equal to 58.14% (49.72% for commercial partnerships) of the capital gains realized. In this event, the relating capital losses would be deductible for a corresponding amount.
Capital gains realized by Italian resident commercial companies subject to IRES, private and public entities and trusts whose sole or principal purpose is to carry out a business activity, are included in their taxable income and are subject to IRES according to the ordinary rules. If the Shares were held and accounted for as fixed financial assets in the three-year period preceding the disposal, the shareholder may elect to spread any realized gain on a straight line basis across the five-year period commencing in the tax year in which the gain is realized and the following four pursuant to article 86(4) of the CITA.
However, under article 87 of the CITA (“participation exemption” regime), capital gains arising from the disposal of the Shares are tax-exempt for 95 percent of such capital gains, whereas the remaining 5% is included in the shareholders’ taxable income and is subject to IRES, if the following conditions are met:
141
If the aforementioned requirements are met, the capital losses made on holdings are not deductible from business income.
Capital losses and negative differences between revenue and costs for shares that do not meet the requirements for participation exemption are not relevant up to the non-taxable amount of dividends, or of accounts thereof, received in the thirty six months prior to their transfer. This provision applies with reference to shares acquired during the 36 month period prior to the realization of capital losses or negative differences, provided that the conditions under (c) and (d) above are met; such a provision does not apply to parties who prepare their financial statements in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board referred to in Regulation (EC) No. 1606/2002 of the European Parliament and Council of July 19, 2002.
Capital losses in excess of Euro 50,000 must be reported to the Italian tax administration in the tax return.
Moreover, the data and the information relating to capital losses in excess of Euro 5,000,000, deriving from the sales of shares accounted for as fixed financial assets, must be included in the recipient’s tax return. Such an obligation does not apply to parties who prepare their financial statements in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Under certain conditions, capital gains on the Shares realized by certain companies and commercial entities are also subject to IRAP, at ordinary rates.
Capital gains realized on the sale or disposal of the Shares by Italian-resident public or private non-commercial entities and trusts are subject to the tax regime described in connection with capital gains realized by Italian resident individual shareholders otherwise than in connection with a business activity.
Capital gains realized by Italian resident pension funds established pursuant to article 17 of Legislative Decree No. 252 of December 5, 2005 are subject to the same tax regime described under the paragraph relating to the taxation regime of dividends received by such funds, above. Subject to certain limitations and requirements (including a minimum holding period), capital gains realized by certain pension funds, not in connection with a Qualified Shareholding, may be exempt from any taxation if the shares meet the requirements set by article 1 (88-114) of the Italian Budget Law for 2017.
Capital gains realized by Italian resident Investment Funds, SICAVs and SICAFs are subject to the same tax regime described under the paragraph relating to the taxation regime of dividends received by such entities, above.
142
Capital gains realized by real estate investment funds and real estate SICAFs are subject to the same tax regime described under the paragraph relating to the taxation regime of dividends received by such entities, above.
Capital gains realized by non-Italian resident shareholders without a permanent establishment in Italy, through which the relevant Shares are held, are subject to the following tax regimes:
The tax regimes described above will not prevent the application, if more favorable to the taxpayer, of any different provisions of any applicable double taxation treaty with Italy. Most double taxation treaties entered into by Italy provide that capital gains realized on the disposal of shares are subject to tax only in the Country of residence of the seller. In such a case, the capital gains realized by non-resident shareholders on the disposal of the Shares will not be subject to tax in Italy. Under article 13(4) of the Italy-U.S. double tax treaty, capital gains realized by treaty entitled U.S. resident shareholders upon disposal of the Shares would be subject to tax only in the U.S.
Article 1(633) of the 2021 Budget Law has introduced favorable tax regime applicable to certain UCIs established outside of Italy according to which capital gains derived from Qualified Shareholdings in Italian tax resident companies are not subject to taxation in Italy, if realized by: (i) foreign UCIs compliant with Directive 2009/65/EC (UCITS Directive), or (ii) foreign UCIs (not compliant with Directive 2009/65/EC) established in an EU Member State or EEA Member State allowing for an adequate exchange of information for tax purposes and whose manager is subject to regulatory supervision in the Country where it is established pursuant to Directive 2011/61/EU (AIFM Directive).
Capital gains realized by non-resident shareholders holding the shareholding through a permanent establishment in Italy are included in the permanent establishment’s overall taxable income and are subject to tax in accordance with the tax regime indicated for capital gains realized by Italian resident companies or commercial entities, above.
Financial Transaction Tax
Article 1(491-500) of Law No. 228 of December 24, 2012 introduced a financial transaction tax (“FTT”) applicable, among others, to the transfers of the ownership of (i) shares issued by Italian joint stock companies (società per azioni), (ii) participating financial instruments (as defined under article 2346(6) of the Italian Civil Code) issued by Italian resident corporations and (iii) securities representing equity investments in Italian resident corporations. The residence of the issuer for the purposes of FTT is the place where the issuer has its registered office.
Since the registered office of Stevanato is in Italy, transfers of ownership of the Shares will be subject to FTT.
The FTT is due by the transferee of the relevant financial instruments and is generally levied by any financial intermediary intervening in the transaction and has to be paid on or before the 16th day of the month following the one in which the ownership was transferred.
The FTT rates are equal to 0.10 percent for transfers of shares executed in regulated stock markets or through multilateral trading facilities and 0.20 percent for all other taxable transfers. Based on the specific FTT regulations, on the assumption that the NYSE is considered a regulated stock market for FTT purposes, the transfer of the Shares should be subject to 0.10 percent FTT tax rate.
143
Shareholders are recommended to consult their independent advisors with respect to the application of FTT.
Stamp Duty
Pursuant to article 13(2bis-2ter) of the Tariff attached to Presidential Decree No. 642 of October 26, 1972, as amended, regulating the Italian stamp duty (imposta di bollo), subject to certain conditions, a stamp duty may be due, at the rate of 0.2 percent on the market value of the Shares, in connection with the periodic reporting communications sent by Italian financial intermediaries to their clients with respect to any financial instruments (such as the Shares), if deposited with an Italian financial intermediary or with an Italian permanent establishment of a foreign financial intermediary. The stamp duty cannot exceed Euro 14,000 for taxpayers other than individuals.
The stamp duty applies to any investor who is a client (as defined in the regulations issued by the Bank of Italy on June 20, 2012) of an entity that exercises in any form a banking, financial or insurance activity within the Italian territory.
Tax on the Value of Financial Activities Held Abroad
Italian resident individuals, certain partnerships (società semplici) and non-commercial entities holding financial activities abroad shall be generally subject to tax on the value thereof (“Ivafe”).
Ivafe applies at a rate of 0.2 percent on the value of the financial activity and is due in proportion to the percentage of ownership and the holding period. The value of financial activity corresponds to the market value at the end of each calendar year (or at the end of the holding period); if it is not available, the relevant value is the nominal or the redemption value.
A tax credit is generally granted for any net worth tax paid abroad by the Italian resident individual in relation to the same financial activities, in an amount not exceeding the Ivafe due.
Details of the financial activities held abroad have to be inserted in the income tax return to be filed in Italy by the Italian resident individuals.
Tax Monitoring Obligations
Individuals, non-commercial entities and certain partnerships (in particular, società semplici or similar partnerships in accordance with article 5 of the TUIR) resident in Italy for tax purposes are required to report in their yearly income tax return, for tax monitoring purposes, the amount of securities and financial instruments (including the Shares) held abroad during a tax year, from which income taxable in Italy may be derived.
In relation to the Shares, such reporting obligation shall not apply if the Shares are not held abroad and, in any case, if the Shares are deposited with an Italian financial intermediary that intervenes in the collection of the relevant income and the intermediary applied the due withholding or substitute tax on any income derived from such Shares.
Inheritance and Gift Tax
Subject to certain exceptions, Italian inheritance and gift tax is generally payable on transfers of assets and rights (including shares) (i) by reason of death or donations by Italian residents, even if the transferred assets are held outside Italy and (ii) by reason of death or donations by non-Italian residents, but limited to transferred assets located in Italy (which are presumed by law to include shares of Italian resident companies).
Subject to certain exceptions, transfers of assets and rights (including the Shares) on death or by gift are generally subject to inheritance and gift tax:
144
If the beneficiary of any such transfer is an individual with a severe disability pursuant to Law No. 104 of February 5, 1992, inheritance or gift tax is applied only on the value of the asset transferred in excess of Euro 1,500,000 at the rates illustrated above, depending on the relationship existing between the deceased or donor and the beneficiary.
U.S. Federal Income Tax Considerations
The following is a summary of U.S. federal income tax considerations generally applicable to the ownership and disposition of Shares by U.S. Holders (as defined below). Unless otherwise noted, this summary addresses only U.S. Holders that hold our Shares as capital assets for U.S. federal income tax purposes. This summary is based on the U.S. Internal Revenue Code of 1986, as amended (the “Code”), U.S. Treasury regulations promulgated thereunder (“Regulations”), judicial decisions, administrative pronouncements, and other applicable authorities, all as in effect as of the date hereof and all of which are subject to change or differing interpretations (possibly with retroactive effect). This summary does not address all aspects of U.S. federal income taxation that may be relevant to a particular holder in light of that holder’s particular circumstances or that may be relevant to certain types of holders subject to special treatment under U.S. federal income tax law, such as:
In addition, this summary does not address any U.S. state or local or non-U.S. tax considerations or any U.S. federal estate, gift, or alternative minimum tax considerations, or the Medicare tax on certain net investment income.
The information set forth below is of a general nature only and is not intended to be tax advice. Each prospective investor should consult its tax advisors with respect to the U.S. federal, state, local and non-U.S. income and other tax considerations of owning and disposing of Shares in light of its particular circumstances.
145
For purposes of this discussion, a “U.S. Holder” is a beneficial owner of Shares that is, for U.S. federal income tax purposes:
If a partnership (or other entity or arrangement treated as a partnership for U.S. federal income tax purposes) is a beneficial owner of our shares, the tax treatment of a partner in the partnership will generally depend upon the status of the partner and the activities of the partnership. Partnerships holding our shares and their partners should consult their tax advisors regarding an investment in our shares.
Distributions
The gross amount of any distributions received by a U.S. Holder on the Shares (including any amounts withheld in respect of Italian withholding taxes) will generally be subject to tax as dividends to the extent paid out of our current or accumulated earnings and profits, as determined for U.S. federal income tax purposes, and will be includible in the gross income of each U.S. Holder on the day actually or constructively received. Distributions in excess of our current and accumulated earnings and profits will be treated as a non-taxable return of capital to the extent of the U.S. Holder’s adjusted tax basis in the Shares and thereafter generally as capital gain. We do not intend to calculate our earnings and profits for U.S. federal income tax purposes, however. Therefore, U.S. Holders should expect that a distribution will generally be treated as a dividend even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above. Any such dividends will not be eligible for the dividends received deduction generally allowed to U.S. corporations under the Code. The following discussion assumes that any dividends will be paid in Euros.
The gross amount of any dividend paid in Euros, including any taxes withheld therefrom, will be included in the gross income of a U.S. Holder in an amount equal to the U.S. Dollar value of the Euros received calculated by reference to the exchange rate in effect on the date the dividend distribution is received, regardless of whether the payment is in fact converted into U.S. Dollars on such date.
If the Euros are converted into U.S. Dollars on the date of receipt, a U.S. Holder should generally not be required to recognize any foreign currency gain or loss in respect of the dividend. If the Euros received are not converted into U.S. Dollars on the date of receipt, a U.S. Holder will have a basis in such Euros equal to their U.S. Dollar value on the date of receipt. Any recognized gain or loss on a subsequent conversion or other disposition of the Euros will be treated as ordinary income or loss, and will generally be income or loss from sources within the United States for foreign tax credit limitation purposes.
Individuals and other non-corporate U.S. Holders may be eligible for reduced rates of taxation on dividends received from a qualified foreign corporation, provided that certain holding period and other requirements are satisfied. A non-U.S. corporation that is not classified as a passive foreign investment company (“PFIC”) with respect to the relevant U.S. Holder for the taxable year in which the dividend is paid or the preceding taxable year is generally treated as a qualified foreign corporation with respect to dividends on shares that are “readily tradable” on an “established securities market” in the United States. The Shares have been approved for listing on the NYSE, which is an established securities market in the United States and are expected to be treated as readily tradable for this purpose. There can be no assurance, however, that the Shares will be considered readily tradable on an established securities market for purposes of these rules in the current year or in future years.
Dividends on the Shares will generally be treated as income from sources outside the United States and will generally constitute passive category income for U.S. foreign tax credit purposes. Depending on the individual facts
146
and circumstances, a U.S. Holder may be eligible, subject to a number of complex limitations, to claim a foreign tax credit not in excess of any applicable treaty rate in respect of any foreign withholding taxes imposed on dividends received on the Shares. A U.S. Holder who does not elect to claim a foreign tax credit for foreign taxes withheld may instead claim a deduction, for U.S. federal income tax purposes, in respect of such withholding, but only for a year in which such U.S. Holder elects to do so for all creditable foreign income taxes. The rules governing the U.S. foreign tax credit are complex and the application thereof depends in large part on the U.S. Holder’s individual facts and circumstances. Accordingly, U.S. Holders should consult their tax advisors regarding the availability of the U.S. foreign tax credit in light of their particular circumstances.
Sale or Other Disposition of Shares
A U.S. Holder will generally recognize gain or loss on the sale or other disposition of Shares in an amount equal to the difference between the amount realized on the disposition (or, if the amount realized is denominated in a foreign currency, the U.S. Dollar equivalent thereof, generally determined by reference to the spot rate of exchange on the date of disposition) and the holder’s adjusted tax basis in such Shares. Any such gain or loss will generally be long-term capital gain or loss if the holder’s holding period for the Shares exceeds one year at the time of disposition and will generally be U.S. source gain or loss for U.S. foreign tax credit purposes. Individuals who are U.S. Holders will generally be subject to U.S. federal income tax on net long-term capital gains at a lower rate than the rate applicable to ordinary income. The deductibility of capital losses is subject to limitations. U.S. Holders should consult their tax advisors regarding the tax consequences to them if a foreign tax is imposed on their disposition of Shares, including with respect to the availability of the foreign tax credit in their particular circumstances.
Passive Foreign Investment Company
A non-U.S. corporation, such as the Company, will be classified as a PFIC for U.S. federal income tax purposes for any taxable year if either (i) 75% or more of its gross income for such year consists of certain types of “passive” income (the “income test”) or (ii) 50% or more of the value of its assets (determined on the basis of a quarterly average) during such year is attributable to assets that produce or are held for the production of passive income (the “asset test”). Passive income generally includes dividends, interest, rents, royalties and net gains from the disposition of passive assets. Passive assets are those which give rise to passive income, and include assets held for investment, as well as cash, assets readily convertible into cash, and (subject to certain exceptions) working capital. The company’s goodwill and other unbooked intangibles are taken into account and may be classified as active or passive depending upon the relative amounts of income generated by the company in each category. We will be treated as owning a proportionate share of the assets and earning a proportionate share of the income of any other corporation in which we own directly or indirectly, or constructively, 25% or more (by value) of its stock.
Based on our income and assets, and the value of our Shares, we do not believe that we were a PFIC for U.S. federal income tax purposes for the taxable year ended December 31, 2022 and we do not expect to become a PFIC for the current taxable year or in any future taxable year. PFIC status is a factual determination, however, and must be made annually after the close of each taxable year, on the basis of the composition of our income and assets. Therefore, there can be no assurance that we were not classified as a PFIC for U.S. federal income tax purposes for the taxable year ended December 31, 2022, or will not be classified as a PFIC for U.S. federal income tax purposes for the current taxable year or in any future taxable year.
If we are a PFIC for any taxable year during which a U.S. Holder holds Shares, the U.S. Holder will be subject to special tax rules with respect to any “excess distribution” that the U.S. Holder receives and any gain that the U.S. Holder realizes from a sale or other disposition (including a pledge) of its Shares, unless the U.S. Holder makes a “mark-to-market” election as discussed below. Distributions received by a U.S. Holder on Shares in a taxable year that exceed 125% of the average annual distributions on the Shares that the U.S. Holder received in the three preceding taxable years or, if shorter, the U.S. Holder’s holding period for the Shares, will be treated as an excess distribution. Under these special tax rules:
147
If we are a PFIC for any taxable year during which a U.S. Holder holds the Shares and any of our non-U.S. subsidiaries are also PFICs, the U.S. Holder will be treated as owning a proportionate amount (by value) of the shares of each such non-U.S. subsidiary classified as a PFIC for purposes of the application of these rules.
If we are a PFIC, certain elections may be available that would result in alternative treatments, such as mark-to-market treatment, of the Shares. such U.S. Holder should consult its tax adviser as to whether a mark-to-market election is available or advisable with respect to the Shares. Because, as a technical matter, however, a mark-to-market election cannot be made for any lower-tier PFICs that we may own, a U.S. Holder may continue to be subject to the PFIC rules with respect to such U.S. Holder’s indirect interest in any such lower-tier PFICs. We do not intend to prepare or provide the information that would enable U.S. Holders to make a qualified electing fund election, which, if available, would result in tax treatment different from the general tax treatment for PFICs discussed above. If we are considered a PFIC, a U.S. Holder also will be subject to annual information reporting requirements.
If we are a PFIC for any taxable year during which a U.S. Holder holds Shares, we will continue to be treated as a PFIC with respect to such U.S. Holder’s Shares unless (i) we cease to be a PFIC and (ii) the U.S. Holder has made a “deemed sale” election under the PFIC rules.
U.S. Holders should consult their tax advisers about the potential application of the PFIC rules to an investment in the Shares.
Foreign Financial Asset Reporting
Certain U.S. Holders are required to report their holdings of certain foreign financial assets, including equity of foreign entities, if the aggregate value of all of these assets exceeds certain threshold amounts. The Shares are expected to constitute foreign financial assets subject to these requirements unless the Shares are held in an account at certain financial institutions. Significant penalties may be imposed for failure to comply with these reporting requirements.U.S. Holders should consult their tax advisors regarding the application of these rules.
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
H. Documents on Display
The SEC maintains an Internet site that contains reports, information statements, and other information regarding issuers that file electronically with the SEC, including Stevanato Group, at http://www.sec.gov. The address of the SEC’s website is provided solely for information purposes and is not intended to be an active link. Reports and other information concerning the business of Stevanato Group may also be inspected at the offices of the New York Stock Exchange, 11 Wall Street, New York, New York 10005. In addition, we make the information filed with or furnished to the SEC available free of charge through our website (www.stevanatogroup.com) or by calling us at +39 049 9318111 as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. The information contained on our website is not a part of this annual report.
As a foreign private issuer, we are exempt under the Exchange Act from, among other things, the rules prescribing the furnishing and content of proxy statements. While we furnish proxy statements to shareholders in accordance with the rules of any stock exchange on which our ordinary shares may be listed in the future, those proxy statements will not conform to Schedule 14A of the proxy rules promulgated under the Exchange Act. Our executive officers, directors
148
and principal shareholders are also exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. Although we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act, we furnish the holders of our ordinary shares with annual reports containing audited financial statements and a report by our independent registered public accounting firm and make available quarterly reports containing selected unaudited financial data for the first three quarters of each fiscal year. The audited financial statements are prepared in accordance with IFRS and include an “Operating and Financial Review and Prospects” section for the relevant periods.
I. Subsidiary Information
Not applicable.
J. ANNUAL REPORT TO SECURITY HOLDERS
The Company intends to submit any annual report provided to security holders in electronic format as an exhibit to a current report on Form 6-K.
ITEM 11. Quantitative and Qualitative Disclosures about Market Risk
Please refer to Note 38 “Qualitative and quantitative information of financial risks” to the Consolidated Financial Statements included elsewhere in this document for details on the market risks that the Group is exposed to.
ITEM 12. Description of Securities Other than Equity Securities
Not applicable.
Not applicable.
Not applicable.
Not applicable.
149
PART II
ITEM 13. Defaults, Dividend Arrearages and Delinquencies
None.
ITEM 14. Material Modifications to the Rights of Security Holders and Use of Proceeds
Material Modifications to the Rights of Security Holders
None.
Use of proceeds
See Item 5 “Operating and Financial Review and Prospects—Liquidity and Capital Resources”.
ITEM 15. Controls and Procedures
Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as that term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (“Exchange Act”)) that are designed to ensure that information required to be disclosed in the Company’s reports under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosures. Any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives.
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2023. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were not effective at the reasonable assurance level as of December 31, 2023, due to the material weaknesses below reported under the section “Management’s annual report on internal control over financial reporting”.
After thoroughly considering these material weaknesses, along with additional analyses and procedures conducted to ensure the compliance of our consolidated financial statements included in this Annual Report on Form 20-F with generally accepted accounting principles, our management believes that our consolidated financial statements present fairly, in all material aspects, our financial position, results of operations, and cash flows for the disclosed period in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board. Furthermore, there have been no changes to the financial statements previously presented by the Company.
Management’s annual report on internal control over financial reporting
Our management, including the Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting (as such term is defined by Rule 13a-15(f) and 15d-15(f) under the Securities Exchange Act). Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the consolidated financial statements for external purposes in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board and includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect our transactions and dispositions of assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board, and that our receipts and expenditures are being made only in accordance with the authorizations of our management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
150
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management has assessed the effectiveness of internal control over financial reporting as of December 31, 2023 based on the criteria established in Internal Control–Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this assessment, our management has concluded that our internal control over financial reporting was not effective as of December 31, 2023, due to the material weaknesses described below.
A material weakness is a deficiency, or combination of control deficiencies in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected in a timely basis.
We did not design and maintain an effective control environment commensurate with our financial reporting requirements. Specifically, we lacked a sufficient complement of personnel with an appropriate level of internal controls knowledge, training and experience commensurate with our financial reporting requirements. Furthermore, we did not design and maintain effective controls in response to the risks of material misstatement as existing controls or the design and implementation of new controls were not sufficient to respond to changes to the risks of material misstatement related to financial reporting.
These material weaknesses contributed to the following additional material weaknesses:
These material weaknesses resulted in a limited number of material adjustments corrected by management in preparing the Company’s 2023 consolidated financial statements prior to issuance. Additionally, these material weaknesses could result in misstatements that would result in a material misstatement of the interim or annual financial statements and disclosures that would not be prevented or detected.
Our independent registered public accounting firm, PricewaterhouseCoopers SpA, has issued an audit report with respect to the effectiveness of our internal control over financial reporting as of December 31, 2023, which appears in Part III, Item 18 of this Annual Report on Form 20-F.
151
Remediation plan
Since the Company became a U.S. listed company, and therefore subject to the reporting requirements of the Sarbanes-Oxley Act, management has been working on strengthening its control environment and its internal control over financial reporting. Activities have included the following areas: i) risk assessment and scoping activities aimed at identifying risks relevant to financial reporting; and ii) formalization of risk and control matrices, including the definition of Information Technology General Controls for the systems considered relevant for the disclosure of our financial statements.
Considering the material weaknesses identified based on the assessment performed as of December 31, 2023, and the commitment the Company has to maintain a robust and effective internal control system, under the supervision of the Audit Committee, the Company’s management will continue implementing and fostering the internal control culture, completing the remedial actions already in place and implementing the remedial actions. Specifically, management has planned the following main measures to continue the process of executing its remediation plan:
The process of designing and maintaining effective internal control over financial reporting is a continuous effort that requires management to anticipate and react to changes in our business, economic, and regulatory environments and to expend significant resources. As we continue to evaluate our internal control over financial reporting, we may take additional actions to remediate the material weaknesses or modify the remediation actions described above.
While we continue to devote significant time and attention to these remediation efforts, the material weaknesses will not be considered remediated until management completes the design and implementation of the actions described above and the controls operate for a sufficient period of time, and management has concluded, through testing, that these controls are effective.
Changes in internal control over financial reporting
There have been no changes in the Company's internal control over financial reporting that have occurred during the period covered by this Annual Report that have materially affected or are reasonably likely to materially affect our internal control over financial reporting.
ITEM 16. RESERVED
Not applicable.
ITEM 16A. Audit Committee Financial Expert
Our board of directors has determined that Fabio Buttignon and William Federici, the chair of the Audit Committee of our board of directors, are each an “Audit Committee financial expert” as defined by Item 16A of Form 20-F. All members of the Audit Committee are independent directors as required by applicable NYSE listing rules and SEC rules. Please see the section entitled “Item 6. Directors, Senior Management and Employees —A. Directors and Senior Management” for more information concerning Fabio Buttignon and William Federici.
152
ITEM 16B. Code of Ethics
Our Code of Business Conduct and Ethics is a code of ethics within the meaning of Item 406(b) of Regulation S-K of the Exchange Act and covers a broad range of matters including the handling of conflicts of interest, compliance issues and other corporate policies such as equal opportunity and non-discrimination standards. Our Code of Business Conduct and Ethics is available in the Investor Relations section of our website at www.stevanatogroup.com.
ITEM 16C. Principal Accountant Fees and Services
Our audit committee charter requires that all audit and non-audit services provided by our independent registered public accounting firm, other than that de minimis non-audit services which may be approved in accordance with applicable rules and regulations, are pre-approved by our audit committee. In particular, pursuant to our audit committee charter, the chairman of the audit committee shall pre-approve all audit services to be provided to Stevanato, whether provided by our independent registered public accounting firm or other firms, and all other services to be provided to Stevanato by the independent registered public accounting firm. Any decision of the chairman of the audit committee to pre-approve audit or non-audit services shall be presented to the audit committee.
The following table represents aggregate fees accrued for professional services rendered by our independent registered public accounting firm for the last two fiscal years. Ernst & Young S.p.A. (EY) served as the Company's statutory auditor and independent registered public accounting firm until Annual General Meeting held on May 24, 2023 when the Company's shareholders approved the appointment of PricewaterhouseCoopers S.p.A. (PwC) for the financial years ending December 31, 2023, 2024 and 2025. The fees were billed in € for the fiscal year ended December 31, 2023 and 2022 by PwC and EY respectively.
|
|
For the Year ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Audit Fees |
|
|
1,323,197 |
|
|
|
1,033,529 |
|
Audit-Related Fees |
|
|
— |
|
|
|
7,000 |
|
Tax Fees |
|
|
— |
|
|
|
— |
|
All Other Fees |
|
|
— |
|
|
|
— |
|
Total |
|
|
1,323,197 |
|
|
|
1,040,529 |
|
Audit Fees
Audit fees consist of the aggregate fee earned by PwC and EY entities for the audit of our consolidated annual financial statements, reviews of interim financial statements and attestation services that are provided in connection with statutory and regulatory filings or engagements.
Audit-Related Fees
Audit-related fees include assurance and related services by the principal accountant that are reasonably related to the performance of the audit or review of the registrant’s financial statements and are not reported as Audit fees in this Item. The fees reported in this category relate to agreed-upon procedures.
Tax Fees
None.
All Other Fees
None.
ITEM 16D. Exemptions from the Listing Standards for Audit Committees
None.
153
ITEM 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
ITEM 16F. Change in Registrant’s Certifying Accountant
As of the date of the Annual General Meeting held on May 24, 2023 (the “Annual General Meeting”), the term of EY appointment as the Company's statutory auditor and independent registered public accounting firm expired. Also at the Annual General Meeting, based on the proposal from the Company and its Audit Committee following a competitive audit tender, which included proposals from both EY and PWC the Company's shareholders approved the appointment of PwC to serve as the Company's statutory auditor and independent registered public accounting firm for the financial years ending December 31, 2023, December 31, 2024 and December 31, 2025. Following the appointment of PwC as described above, from the date of the Annual General Meeting EY S.p.A. was dismissed by the Company and ceased to be our independent registered public accounting firm for our consolidated financial statements included in our reports on Form 20-F, and PwC is our independent registered public accounting firm for our consolidated financial statements to be included in our reports on Form 20-F.
The reports of EY on our financial statements for the past two years did not contain an adverse opinion or a disclaimer of opinion and were not qualified or modified as to uncertainty, audit scope or accounting principles.
In connection with the audits of our financial statements for each of the two fiscal years ended December 31, 2022 and in the subsequent interim period through the date of the Annual General Meeting, (i) there were no disagreements with EY on any matter of accounting principles or practices, financial statement disclosure or auditing scope and procedures which if not resolved to the satisfaction of EY, would have caused EY to make reference to the matter in their report; and (ii) there were no “reportable events” as described in Item 16F(a)(1)(v) of Form 20-F.
The Company provided EY with a copy of the foregoing disclosure and has requested EY to furnish a letter addressed to the SEC stating whether they agree with the above statements. A copy of that letter dated March 7, 2024 is being filed as Exhibit 15.1 to this Form 20-F.
During the two fiscal years ended December 31, 2022 and the subsequent interim period through the date of the Annual General Meeting, neither the Company nor anyone acting on the Company’s behalf consulted with PwC regarding: (1) the application of accounting principles to a specific transaction, either completed or proposed; (2) the type of audit opinion that might be rendered on the Company’s financial statements and neither a written report nor oral advice was provided to the Company that PwC concluded was an important factor considered by the Company in reaching a decision as to any accounting, auditing or financial reporting issue; or (3) any matter that was the subject of a disagreement (as described in Item 16F(a)(1)(iv) of Form 20-F and the related instructions thereto).
During the two financial years ended December 31, 2022 and the subsequent interim period through the date of the Annual General Meeting, there have not been any “reportable events” (as described in Item 16F(a)(1)(v) of Form 20-F) as it relates to PwC.
ITEM 16G. Corporate Governance
Stevanato Group S.p.A. is a company organized under the laws of Italy and qualifies as a foreign private issuer under the rules and regulations of the SEC and the listing standards of the NYSE. In accordance with the NYSE rules related to corporate governance, listed companies that are foreign private issuers are permitted to follow home‑country practice in some circumstances in lieu of the provisions of the corporate governance rules contained in Section 303.A of the NYSE Listed Company Manual that are otherwise applicable to listed companies. In addition, we must disclose any significant ways in which our corporate governance practices differ from those followed by U.S. companies listed on the NYSE.
In addition to the above, Stevanato Group S.p.A. is exempt from certain other NYSE corporate governance requirements pursuant to its status of “controlled company”. Stevanato Holding S.r.l. directly controls a majority of the voting power of our issued and outstanding shares and we are therefore a controlled company within the meaning of the NYSE Listed Company Manual. Under these standards, a company of which more than 50% of the voting power is held by another person or group of persons acting together is a controlled company and may elect not to comply with certain NYSE corporate governance requirements, including the requirements that: (i) a majority of the
154
board of directors consist of independent directors, (ii) the nominating and governance committee be composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities, (iii) the compensation committee be composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities, and (iv) there be an annual performance evaluation of the nominating and corporate governance and compensation committees.
As a result of the foregoing exemptions, our shareholders may not have the same protections afforded to shareholders of companies that are subject to all of the NYSE corporate governance requirements.
Please refer to “Item 6 Directors, Senior Management and Employees” and “Item 10.B Additional Information – Memorandum of Association and By-Laws” for further information.
ITEM 16H. Mine Safety Disclosure
None.
ITEM 16I. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
None.
ITEM 16J. INSIDER TRADING POLICIES
The registrant has
ITEM 16K. CYBERSECURITY
We believe cybersecurity is key to the Company achieving its strategic goals and objectives. Based on the nature of our business and the industry in which we operate, we are faced with a variety of cybersecurity threats including phishing emails, ransomware attacks, malicious attachments, social engineering attacks and denial of service attacks, among others. Our customers, suppliers, subcontractors and partners face similar cybersecurity threats, and a cybersecurity incident impacting us or any of these entities could materially adversely affect our operations, performance and results of operations.
Our information security organization has implemented a governance structure and processes to assess, identify, manage and report cybersecurity risks. We engage third-party service providers to conduct evaluations of our security controls, including testing both the design and operational effectiveness of security controls.
In the event of an incident, we intend to follow our incident management procedures, which outline the steps to be followed from incident detection to mitigation, recovery and notification, including notifying functional areas (e.g., legal, compliance and internal audit), as well as senior leadership and the Board, as appropriate.
On a regular basis, the Company analyzes its internet-based services and performs penetration tests and attack simulations to assess the protection and the detection capabilities. The cybersecurity compliance status of assets is centrally evaluated across the Company's global sites and business and operational functions. Results are shared within the Company's relevant business units and across global functions. The Company implements corrective measures and improvement actions in response to these processes, as appropriate. Data classification and protection tools are in place, such as the implementation of a specific process and technology aimed at detecting and responding to abnormal data flows.
Governance
The Board of Directors and our Audit Committee oversee management’s processes for identifying and mitigating risks, including cybersecurity risks, to help align our risk exposure with our strategic objectives. Senior leadership, including our Chief Information Officer (CIO), have developed a process to regularly brief the Audit Committee and Board of Directors on our cybersecurity and information security policies and procedures, and the Board of Directors will be apprised of cybersecurity incidents deemed to have a potential material impact on the Company.
155
Our information security organization, led by our CIO, is responsible for our overall information security strategy, policy, cyber threat detection and response, cyber architecture and processes for the security of our enterprise network, information assets and medical device technologies. The CIO’s organization monitors and manages, and works to identify and assess, cybersecurity risk through various technologies, resources, processes and policies that are regularly updated to align with the changing threat landscape, our evolving business needs as well as global regulatory requirements. The current CIO and his organization, comprised of both internal and external resources, have extensive information technology and program management experience. This organization manages and is continually enhancing and building an enterprise security structure with the ultimate goal of preventing cybersecurity incidents to the extent feasible, while simultaneously engaging in efforts to minimize the business impact should an incident occur.
Cybersecurity risks and threats, including as a result of any previous cybersecurity incidents, have not materially impacted and are not reasonably expected to materially impact us or our operations to date. However, we recognize the ever-evolving cyber risk landscape and cannot provide any assurances that we will not be subject to a material cybersecurity incident in the future. See Item 1A, “Risk Factors” for a discussion of cybersecurity risks.
156
PART III
ITEM 17. Financial Statements
Please see “Item 18. Financial Statements” below.
ITEM 18. Financial Statements
The financial statements and the related notes required by this Item 18 are included in this annual report beginning on page F-1.
ITEM 19. Exhibits
Exhibit Number |
Description of Document |
1.1 |
|
1.2* |
|
2.1* |
|
4.1** |
|
4.2* |
|
4.3** |
|
4.4** |
|
4.5 |
|
4.6 |
|
4.7** |
|
4.8** |
|
4.9** |
|
4.10** |
|
8.1* |
|
11.1* |
|
11.2* |
|
12.1* |
|
12.2* |
|
13.1* |
Certification of Franco Moro furnished pursuant to 17 CFR 240.13a-14(b) and 18 U.S.C. 1350. |
13.2* |
Certification of Marco Dal Lago furnished pursuant to 17 CFR 240.13a-14(b) and 18 U.S.C. 1350. |
15.1* |
Letter from EY regarding Change in Registrant's Certifying Accounting Firm. |
23.1* |
Consent of Independent Registered Public Accounting Firm (EY S.p.A.) |
23.2* |
Consent of Independent Registered Public Accounting Firm (PricewaterhouseCoopers S.p.A.) |
97* |
157
101.INS |
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document. |
101.SCH |
Inline XBRL Taxonomy Extension Schema with Embedded Linkbase Documents |
104 |
Cover Page Interactive Data File (embedded within the Inline XBRL document). |
(1) Exhibits other than those listed above are omitted when in the opinion of Stevanato Group S.p.A. they are either not applicable or not material. * Furnished herewith. |
|
** Portions of this exhibit have been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K. |
|
158
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
|
Stevanato Group S.p.A. |
||
|
|
||
|
By: |
/s/ Franco Moro |
|
|
|
Name: |
Franco Moro |
|
|
Title: |
Chief Executive Officer |
Date: March 7 , 2024 |
|
|
|
159
INDEX TO THE CONSOLIDATED FINANCIAL STATEMENTS OF STEVANATO GROUP S.P.A.
Consolidated Financial Statements at December 31, 2023 and 2022, and for the years ended December 31, 2023, 2022 and 2021
Report of Independent Registered Public Accounting Firm (PCAOB ID: 000 |
F-1 |
Report of Independent Registered Public Accounting Firm (PCAOB ID: |
F-4 |
F-5 |
|
F-6 |
|
F-7 |
|
F-8 |
|
F-10 |
|
F-12 |
160
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Stevanato Group SpA
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated statement of financial position of Stevanato Group SpA and its subsidiaries (the “Company”) as of December 31, 2023, and the related consolidated statements of income, of comprehensive income, of changes in equity and of cash flows for the year then ended, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2023, and the results of its operations and its cash flows for the year then ended in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board. Also in our opinion, the Company did not maintain, in all material respects, effective internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO because material weaknesses in internal control over financial reporting existed as of that date related to the Company not designing and maintaining (i) an effective control environment due to an insufficient complement of personnel with an appropriate level of internal controls knowledge, training and experience commensurate with its financial reporting requirements; (ii) effective controls in response to the risks of material misstatement as existing controls or the design and implementation of new controls were not sufficient to respond to changes to the risks of material misstatement related to financial reporting; (iii) formal procedures and controls over substantially all accounts and disclosures, including segregation of duties, adequate controls related to the reliability of system-generated information used in the controls, and the preparation and review of account reconciliations and journal entries; (iv) effective controls over the period-end financial reporting process to achieve complete, accurate, and timely financial accounting, reporting and disclosures, including the classification of various accounts in the financial statements; and (v) effective controls over information technology (“IT”) general controls for information systems that are relevant to the preparation of the consolidated financial statements, including effective controls over program change management, user access and computer operations.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. The material weaknesses referred to above are described in Management's annual report on internal control over financial reporting appearing under Item 15. We considered these material weaknesses in determining the nature, timing, and extent of audit tests applied in our audit of the 2023 consolidated financial statements, and our opinion regarding the effectiveness of the Company’s internal control over financial reporting does not affect our opinion on those consolidated financial statements.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in management's report referred to above. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
F-1
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audit of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Consolidated Financial Statements - Impact of Personnel and Controls Related to Financial Reporting
The completeness and accuracy of the consolidated financial statements, including the financial condition, results of operations and cash flows, is dependent on, in part, management’s ability to (i) maintain a sufficient complement of personnel with an appropriate level of internal controls knowledge, training and experience commensurate with its financial reporting requirements; (ii) design and maintain effective controls to respond to changes to the risks of material
F-2
misstatement related to financial reporting; (iii) design and maintain formal procedures and controls over substantially all accounts and disclosures, including segregation of duties, adequate controls related to the reliability of system-generated information used in the controls, and the preparation and review of account reconciliations and journal entries; (iv) design and maintain effective controls over the period-end financial reporting process to achieve complete, accurate, and timely financial accounting, reporting and disclosures, including the classification of various accounts in the financial statements; and (v) design and maintain effective controls over information technology (“IT”) general controls for information systems that are relevant to the preparation of the consolidated financial statements, including effective controls over program change management, user access and computer operations.
The principal considerations for our determination that performing procedures relating to the consolidated financial statements - impact of personnel and controls related to financial reporting is a critical audit matter are the high degree of auditor judgment, subjectivity and effort in performing procedures and evaluating audit evidence related to business processes that affect substantially all financial statement account balances and disclosures. As described in the “Opinions on the Financial Statements and Internal Control over Financial Reporting” section, material weaknesses were identified as of December 31, 2023 related to (i) an insufficient complement of personnel with an appropriate level of internal controls knowledge, training and experience commensurate with the Company’s financial reporting requirements; (ii) controls in response to the risks of material misstatement as existing controls or the design and implementation of new controls were not sufficient to respond to changes to the risks of material misstatement related to financial reporting; (iii) formal procedures and controls over substantially all accounts and disclosures, including segregation of duties, adequate controls related to the reliability of system-generated information used in the controls, and the preparation and review of account reconciliations and journal entries; (iv) controls over the period-end financial reporting process to achieve complete, accurate, and timely financial accounting, reporting and disclosures, including the classification of various accounts in the financial statements; and (v) controls over IT general controls for information systems that are relevant to the preparation of the consolidated financial statements, including effective controls over program change management, user access and computer operations.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included, among others, evaluating and determining the nature and extent of audit procedures performed and evidence obtained that are responsive to the material weaknesses identified. These procedures also included (i) testing the completeness and accuracy of period-end financial reporting, including the classification and presentation of accounts and disclosures; and (ii) manually testing the completeness and accuracy of system reports or other information generated by the Company’s information systems.
/s/
March 7, 2024
We have served as the Company’s auditor since 2023.
F-3
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of
Stevanato Group S.p.A.
Opinion on the Financial Statements
We have audited the accompanying consolidated statement of financial position of Stevanato Group S.p.A. (the Company) as of December 31, 2022, the related consolidated income statements, consolidated statements of comprehensive income, changes in equity and cash flows for each of the two years in the period ended December 31, 2022, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2022, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2022, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/
We served as the Company’s auditor from 2017 to 2023.
March 2, 2023
F-4
Stevanato Group S.p.A.
Consolidated income statement
for the years ended December 31, 2023, 2022 and 2021
|
|
|
|
For the year ended December 31, |
|
|||||||||
|
|
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
|
|
(EUR thousand) |
|
|||||||||
|
|
Notes |
|
|
|
|
|
|
|
|
|
|||
Revenue |
|
6 |
|
|
|
|
|
|
|
|
|
|||
Cost of sales |
|
7 |
|
|
|
|
|
|
|
|
|
|||
Gross Profit |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||
Other operating income |
|
8 |
|
|
|
|
|
|
|
|
|
|||
Selling and marketing expenses |
|
9 |
|
|
|
|
|
|
|
|
|
|||
Research and development expenses |
|
9 |
|
|
|
|
|
|
|
|
|
|||
General and administrative expenses |
|
9 |
|
|
|
|
|
|
|
|
|
|||
Operating Profit |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||
Finance income |
|
11 |
|
|
|
|
|
|
|
|
|
|||
Finance expense |
|
12 |
|
|
|
|
|
|
|
|
|
|||
Share of profit of an associate |
|
11 |
|
|
|
|
|
|
|
|
|
|||
Profit Before Tax |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||
Income taxes |
|
14 |
|
|
|
|
|
|
|
|
|
|||
Net Profit |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||
Net Profit attributable to: |
|
|
|
|
|
|
|
|
|
|
|
|||
Equity holders of the parent |
|
|
|
|
|
|
|
|
|
|
|
|||
Non-controlling interests |
|
35 |
|
|
|
|
|
|
|
|
( |
) |
||
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||
Earnings per share |
|
|
|
|
|
|
|
|
|
|
|
|||
Basic earnings per common share (in EUR) |
|
15 |
|
|
|
|
|
|
|
|
|
|||
Diluted earnings per common share (in EUR) |
|
15 |
|
|
|
|
|
|
|
|
|
|||
The accompanying notes are an integral part of the Consolidated Financial Statements
F-5
Stevanato Group S.p.A.
Consolidated statement of comprehensive income
for the years ended December 31, 2023, 2022 and 2021
|
|
|
|
For the year ended December 31, |
|
|||||||||
|
|
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
|
|
(EUR thousand) |
|
|||||||||
|
|
Notes |
|
|
|
|
|
|
|
|
|
|||
Net Profit |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||
Gains/(losses) from remeasurement of employee defined benefit plans |
|
28 |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
Gains/(losses) from remeasurement of the agent termination plan |
|
30 |
|
|
|
|
|
|
|
|
|
|||
Tax effect relating to those components of OCI |
|
14 |
|
|
|
|
|
( |
) |
|
|
|
||
Other comprehensive income (loss) that will not be classified subsequently to profit or loss |
|
|
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Exchange difference on translation of foreign operations |
|
24 |
|
|
|
|
|
|
|
|
|
|||
Changes in the fair value of cash flow hedging instruments |
|
27 |
|
|
( |
) |
|
|
|
|
|
|
||
Changes in the time value element - cost of hedge |
|
27 |
|
|
|
|
|
( |
) |
|
|
— |
|
|
Tax effect relating to those components of OCI |
|
14 |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Other comprehensive income that might be classified subsequently to profit or loss |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||
Total other comprehensive income, net of tax |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||
Total Comprehensive Income |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||
Attributable to: |
|
|
|
|
|
|
|
|
|
|
|
|||
Equity holders of the parent |
|
|
|
|
|
|
|
|
|
|
|
|||
Non-controlling interests |
|
|
|
|
|
|
|
|
|
|
( |
) |
||
|
|
|
|
|
|
|
|
|
|
|
|
|||
The accompanying notes are an integral part of the Consolidated Financial Statements
F-6
Stevanato Group S.p.A.
Consolidated statement of financial position
at December 31, 2023 and 2022
|
|
|
|
At December 31 |
|
|
At December 31 |
|
||
|
|
|
|
2023 |
|
|
2022 |
|
||
|
|
|
|
(EUR thousand) |
|
|||||
Assets |
|
Notes |
|
|
|
|
|
|
||
Non-current assets |
|
|
|
|
|
|
|
|
||
Goodwill |
|
16 |
|
|
|
|
|
|
||
Intangible assets |
|
17 |
|
|
|
|
|
|
||
Right of use assets |
|
34 |
|
|
|
|
|
|
||
Property, plant and equipment |
|
18 |
|
|
|
|
|
|
||
Financial assets - investments FVTPL |
|
|
|
|
|
|
|
|
||
Other non-current financial assets |
|
19 |
|
|
|
|
|
|
||
Deferred tax assets |
|
14 |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Current assets |
|
|
|
|
|
|
|
|
||
Inventories |
|
20 |
|
|
|
|
|
|
||
Contract assets |
|
21 |
|
|
|
|
|
|
||
Trade receivables |
|
21 |
|
|
|
|
|
|
||
Other current financial assets |
|
19 |
|
|
|
|
|
|
||
Tax receivables |
|
22 |
|
|
|
|
|
|
||
Other receivables |
|
23 |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Total assets |
|
|
|
|
|
|
|
|
||
Equity and liabilities |
|
|
|
|
|
|
|
|
||
Equity |
|
|
|
|
|
|
|
|
||
Share capital |
|
24 |
|
|
|
|
|
|
||
Reserves and retained earnings |
|
24 |
|
|
|
|
|
|
||
Net profit attributable to equity holders of the parent |
|
24 |
|
|
|
|
|
|
||
Equity attributable to equity holders of the parent |
|
|
|
|
|
|
|
|
||
Non-controlling interests |
|
35 |
|
|
|
|
|
( |
) |
|
Total equity |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Non-current liabilities |
|
|
|
|
|
|
|
|
||
Non-current financial liabilities |
|
26, 34 |
|
|
|
|
|
|
||
Employees benefits |
|
28 |
|
|
|
|
|
|
||
Non-current provisions |
|
30 |
|
|
|
|
|
|
||
Deferred tax liabilities |
|
14 |
|
|
|
|
|
|
||
Non-current advances from customers |
|
33 |
|
|
|
|
|
— |
|
|
Other non-current liabilities |
|
31 |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Current liabilities |
|
|
|
|
|
|
|
|
||
Current financial liabilities |
|
26, 34 |
|
|
|
|
|
|
||
Current provisions |
|
30 |
|
|
|
|
|
— |
|
|
Trade payables |
|
32 |
|
|
|
|
|
|
||
Contract liabilities |
|
33 |
|
|
|
|
|
|
||
Advances from customers |
|
33 |
|
|
|
|
|
|
||
Tax payables |
|
22 |
|
|
|
|
|
|
||
Other liabilities |
|
32 |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Total liabilities |
|
|
|
|
|
|
|
|
||
Total equity and liabilities |
|
|
|
|
|
|
|
|
||
The accompanying notes are an integral part of the Consolidated Financial Statements
F-7
Stevanato Group S.p.A.
Consolidated statement of changes in equity
for the years ended December 31, 2023, 2022 and 2021
|
|
Notes |
|
Share |
|
|
Share |
|
|
Treasury |
|
|
Cash flow |
|
|
Cost of hedging reserve |
|
|
Reserve for |
|
|
Foreign |
|
|
Retained |
|
|
Equity |
|
|
Non- |
|
|
Total |
|
|||||||||||
|
|
|
|
(EUR thousand) |
|
|||||||||||||||||||||||||||||||||||||||||
At January 1, 2021 |
|
|
|
|
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
Other comprehensive income |
|
24 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
( |
) |
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|||||
Net profit |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|||
Total comprehensive income |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|||||
Dividends |
|
25 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
Capital increase |
|
24 |
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||||
Transaction costs on capital increase |
|
24 |
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
Taxes relating to capital increase costs |
|
24 |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|||
Other |
|
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|||
Total effects |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
|
||||
At December 31, 2021 |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|||||
Other comprehensive income |
|
24 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
||||||
Net profit |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total comprehensive income |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Dividends |
|
25 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
Share-based incentive plans |
|
24 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|||
Total effects |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
At December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||||
Other comprehensive income |
|
24 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
|
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
|
||||
Net profit |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
The accompanying notes are an integral part of the Consolidated Financial Statements
F-8
Total comprehensive income |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Dividends |
|
25 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
Share-based incentive plans |
|
24 |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
||||
Acquisition of non-controlling interests |
|
24 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
Other |
|
24 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|||
Total effects |
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
( |
) |
||
At December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the Consolidated Financial Statements
F-9
Stevanato Group S.p.A.
Consolidated statement of cash flows
for the years ended December 31, 2023, 2022 and 2021
|
|
|
|
For the year ended December 31, |
|
|||||||||
|
|
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
|
|
(EUR thousand) |
|
|||||||||
|
|
Notes |
|
|
|
|
|
|
|
|
|
|||
Operating activities |
|
|
|
|
|
|
|
|
|
|
|
|||
Profit before tax |
|
|
|
|
|
|
|
|
|
|
|
|||
Adjustments: |
|
|
|
|
|
|
|
|
|
|
|
|||
- depreciation and impairment of property, plant and equipment |
|
10 |
|
|
|
|
|
|
|
|
|
|||
- amortization of intangible assets and right of use assets |
|
10 |
|
|
|
|
|
|
|
|
|
|||
- allowance for doubtful accounts |
|
21 |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
- net interest expense/(income) |
|
11, 12 |
|
|
|
|
|
|
|
|
( |
) |
||
- share of profit or loss of associated companies |
|
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
- (gain) from the disposal of non-current assets |
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Change in other provisions |
|
|
|
|
|
|
|
( |
) |
|
|
|
||
Change in employee benefits |
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Other non-cash expenses, net |
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Working capital changes net of the effects from purchase of controlled entity: |
|
|
|
|
|
|
|
|
|
|
|
|||
- inventories and contract assets |
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
- trade receivables and other assets |
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
- trade payables, contract liabilities, advances and other liabilities |
|
|
|
|
|
|
|
|
|
|
|
|||
Interest paid |
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Interest received |
|
|
|
|
|
|
|
|
|
|
|
|||
Income tax paid |
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Net Cash Flows from operating activities |
|
|
|
|
|
|
|
|
|
|
|
|||
Cash Flow from investing activities |
|
|
|
|
|
|
|
|
|
|
|
|||
Purchase of property, plant and equipment |
|
18 |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Proceeds from sale of property, plant and equipment |
|
18 |
|
|
|
|
|
|
|
|
|
|||
Purchase of intangible assets |
|
17 |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Proceeds from sale of associated companies |
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
(Increase)/ decrease of financial assets |
|
|
|
|
( |
) |
|
|
|
|
|
|
||
Proceeds from life insurance policies redemption |
|
19 |
|
|
|
|
|
— |
|
|
|
— |
|
|
Business combination - net of cash acquired |
|
4 |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
Net Cash Flows used in investing activities |
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Cash Flow from financing activities |
|
|
|
|
|
|
|
|
|
|
|
|||
Net proceeds from IPO |
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
Acquisition of non-controlling interests |
|
35 |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
Payment of financial payables for shares acquisition |
|
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Dividends paid |
|
25 |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Payment of principal portion of lease liabilities |
|
34 |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Proceeds from borrowings |
|
26 |
|
|
|
|
|
|
|
|
|
|||
Repayments of borrowings |
|
26 |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Decrease in other current financial activities |
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
Net Cash Flows from/(used in) financing activities |
|
|
|
|
|
|
|
( |
) |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|||
Net change in cash and cash equivalents |
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
Net foreign exchange differences on cash and cash equivalents |
|
|
|
|
( |
) |
|
|
|
|
|
|
||
Cash and cash equivalents at January 1 |
|
|
|
|
|
|
|
|
|
|
|
|||
Cash and cash equivalents at December 31 |
|
|
|
|
|
|
|
|
|
|
|
|||
The accompanying notes are an integral part of the Consolidated Financial Statements
F-10
Non-cash investing activities are related to:
- the grant of land (Note 18);
- the investments in property, plant and equipment not paid at December 31, 2023 (Note 18);
- the acquisition of right-of-use assets respectively (Note 34).
Other non-cash expenses, net are mainly related to:
- the change in the cash flow hedge reserve;
- the change in the fair value of derivative instruments;
- the change in equity reserves primarily related to share-based compensation;
- foreign currency unrealized gain and losses.
The accompanying notes are an integral part of the Consolidated Financial Statements
F-11
Stevanato Group S.p.A.
Notes to the consolidated financial statements
1. Corporate information
Stevanato Group S.p.A. (herein referred to as the “Company” and together with its subsidiaries the “Group”) is headquartered in Italy and its registered office is located in via Molinella 17, Piombino Dese (Padua, Italy). The Group is active in the design, production and distribution of products and processes to provide integrated solutions for the bio-pharma and healthcare industries, leveraging our ongoing investment in the business to drive organic growth as well as selected acquisition of skills and new technologies to become a global player in the bio-pharma industry. Principal products are containment solutions, drug delivery systems, medical devices, diagnostic, analytical services, visual inspection machines, assembling and packaging machines, and glass forming machines.
The Group has nine production plants for manufacturing and assembly of bio-pharma and healthcare products (in Italy, Germany, Slovakia, Brazil, Mexico, China, United States), six plants for the production of machinery and equipment (in Italy and Denmark), two sites for analytical services (in Italy and United States) and two commercial offices (in Japan and the United States). The Group is expanding its global capacity primarily for its high-value solutions products. In the United States, the Group is advancing the build out of its new EZ-fill® manufacturing hub in Fishers, Indiana. The plant launched customer validation and expects to start commercial operations in 2024. In Italy, the new facility in Piombino Dese has started commercial production while in Latina the Group launched commercial production in the fourth quarter of 2023. In 2023, the Group elected to slow down its capacity expansion in China in order to focus efforts and resources on the ramp up of its manufacturing facilities in the U.S. and Italy where demand outpaced initial expectations. On November 8, 2023, we acquired Perugini S.r.l., an Italian company specialized in the manufacturing of consumables and mechanical components for industrial machines. The acquisition of Perugini will support the Group's efforts in the ongoing integration of critical technologies and processes into the Group’s production process.
The global footprint allows the Group to sell products and provide services in more than 70 countries worldwide.
Stevanato Group S.p.A. is controlled by Stevanato Holding S.r.l. which holds
On July 16, 2021, Stevanato Group's shares began trading on the New York Stock Exchange under the symbol STVN.
2. Significant accounting policies
2.1 Basis of preparation
The consolidated financial statements comprised the financial statements of the Company and its subsidiaries as at December 31, 2023 and 2022, and for the years ended December 31, 2023, 2022 and 2021. The consolidated financial statements were authorized for issuance by resolution of the Board of Directors on March 5, 2024.
The consolidated financial statements of the Group have been prepared in accordance with the International Financial Reporting Standards as issued by the International Accounting Standards Board (IFRS).
The accounting policies stated below have, unless otherwise stated, been applied consistently over all periods presented in the consolidated financial statements. The Group’s accounting policies have been applied consistently by the Group’s companies.
The consolidated financial statements are composed of a consolidated income statement, a consolidated statement of comprehensive income, a consolidated statement of financial position, a consolidated statement of changes in equity, a consolidated statement of cash flows and the accompanying notes (the “Consolidated Financial Statements”).
The Group presents its consolidated income statement using the function of expense method reflecting the practice in the industry in which the Group operates.
The Group presents current and non-current assets and liabilities as separate classifications in its consolidated statement of financial position.
F-12
The statement of cash flows has been prepared using the “indirect method” allowed by IAS 7 – Cash Flow statements. Starting in 2023, the Group disaggregates the changes in other provisions and the change in employee benefits in the consolidated statements of cash flows. This information was previously presented on a net basis.
The consolidated financial statements have been prepared on a historical cost basis, modified as required for the measurement of certain financial instruments at their fair value.
The consolidated financial statements are presented in Euro, the Group’s presentation currency, which is also the functional currency of the Company and the primary economic environment in which the entity operates. The consolidated financial statements values are rounded to the nearest thousand.
The consolidated financial statements are prepared on a going concern basis. Management believes that there are no financial or other indicators presenting material uncertainties that may cast significant doubt upon the Group’s ability to meet its obligations in the foreseeable future, and not less than one year after the date the consolidated financial statements are approved to be issued.
2.2 Basis of consolidation
Subsidiaries
Subsidiaries are any entities over which the Group has control. The Group controls an entity when the Group is exposed to, or has the rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. Power is generally presumed with an ownership of more than one-half of the voting rights. The existence and effect of potential voting rights that are currently exercisable or convertible are considered when assessing whether the Group controls another entity. The Group re-assesses whether or not it controls an investee if facts and circumstances indicate that there are changes to one or more of the three elements of control.
The Group initially recognizes any non-controlling interests (“NCI”) at fair value or at the non-controlling interest’s share of the recognized amounts of the acquiree’s identifiable net assets. Net profit or loss and each component of other comprehensive income/ (loss) are attributed to the owners of the parent and to the non-controlling interests. Total comprehensive income/ (loss) of subsidiaries is attributed to owners of the parent and to the non-controlling interests even if this results in the non-controlling interests having a deficit balance.
Subsidiaries are fully consolidated from the date on which control is obtained by the Group. The acquisition of further shares in subsidiaries and any sale of shares which do not lead to loss of control are accounted for as transactions between shareholders; as such, the accounting effects of such operations are reflected directly in the Group equity. If the Group loses control over a subsidiary, it derecognizes the related assets (including goodwill), liabilities, non-controlling interest and other components of equity, while any resultant gain or loss is recognized in profit or loss. Any investment retained is recognized at fair value.
Business Combination
The Group's business combinations are accounted for in accordance with the purchase method set out in IFRS 3 - Business Combinations.
Based on the purchase method, the cost of the business combination is allocated to the identifiable acquired net assets, at the acquisition date, through the fair value measurement of the assets acquired and liabilities and contingent liabilities assumed, and goodwill is recognised to the extent of the excess of the business combination cost over the acquirer's interest in the net fair value of the identifiable assets, liabilities and contingent liabilities recognised. If the initial accounting for a business combination can be determined only provisionally, adjustments to the values initially attributed are made within twelve months of the acquisition date. Non-controlling interests are recognised at the fair value of the net acquired assets.
Associates
These are companies in which the Group has a significant influence over their financial and operating policies and which are neither subsidiaries nor joint ventures. The consolidated financial statements show the Group's portion of results of the associated companies, accounted for using the equity method, starting from the date when the significant influence began. Under the equity method, the investments are initially recognized at cost and adjusted thereafter to recognize the Group’s
F-13
share of the profit/ (loss) and other comprehensive income/ (loss) of the investee. The Group’s share of the investee’s profit/ (loss) is recognized in the consolidated income statement.
When significant influence over an associate is lost as a result of a full or partial disposal, the Group derecognises that associate and recognises in profit or loss the difference between, on the one hand, the sum of the proceeds received plus the fair value of any retained interest and, on the other hand, the carrying amount of the investment in the associate at the date significant influence is lost.
Consolidation of foreign companies
The functional currency of the Group’s entities is the currency of their primary economic environment. All the assets and liabilities of foreign companies that report in a currency other than the Euro and which fall within the scope of consolidation are translated into Euro using the exchange rate at the end of the reporting period. Income and costs are translated using average rates for the reporting period. The exchange differences arising on translation for consolidation are recognized in “Foreign currency translation reserve” under consolidated net equity. The difference arising in the year is recognized in OCI. On disposal of a foreign operation, the component of net equity relating to that particular foreign operation is reclassified to profit or loss.
Transactions eliminated upon consolidation
All transactions and balances between Group companies and all unrealized gains and losses arising on intercompany transactions are eliminated on consolidation.
Transactions in foreign currency
Transactions in foreign currencies are initially recorded by the Group’s entities at their respective functional currency spot rates at the date the transaction first qualifies for recognition. Monetary assets and liabilities denominated in foreign currencies at the balance sheet date are translated at the foreign currency exchange rate prevailing at that date.
Exchange differences arising on the extinguishment of monetary items or their translation at different rates to those used for their translation upon initial recognition or in previous financial statements are recorded in the income statement. Non-monetary items that are measured in terms of historical cost in a foreign currency are translated using the exchange rates at the dates of the initial transactions.
The principal foreign currency exchange rates used to translate other currencies into Euro were as follows:
COUNTRY |
|
ISO |
|
Average for the |
|
|
At |
|
|
Average for the |
|
|
At |
|
|
Average for the |
|
|
At |
|
||||||
|
|
|
|
2023 |
|
|
2023 |
|
|
2022 |
|
|
2022 |
|
|
2021 |
|
|
2021 |
|
||||||
CHINA |
|
CNY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
UNITED STATES |
|
USD |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
MEXICO |
|
MXN |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
DENMARK |
|
DKK |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
BRAZIL |
|
BRL |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
SWITZERLAND |
|
CHF |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
JAPAN |
|
JPY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
2.3 Main accounting policies, estimates and assumptions
Goodwill
Goodwill represents the excess of the consideration transferred over the fair value of net assets of businesses acquired. Goodwill is initially measured at cost and after initial recognition, goodwill is measured at cost less any accumulated impairment losses. For the purpose of impairment testing goodwill acquired in a business combination is, from the acquisition date, allocated to each of the Group’s cash-generating units (CGU) that are expected to benefit from the combination.
F-14
Goodwill is tested for impairment annually or more frequently if events or changes in circumstances indicate that it might be impaired.
In accordance with IAS 36 - Impairment of assets, an impairment loss is recognized if the recoverable amount is lower than the carrying amount. An impairment loss recognized for goodwill cannot be reversed in a subsequent period.
Fair Value Measurement
In accordance with IFRS 13 – Fair Value Measurement, the Group measures certain financial instruments such as derivatives and non-financial assets, at fair value at each balance sheet date. Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
IFRS 13 establishes a three-level hierarchy that categorizes the inputs to the valuation techniques used to measure fair value by giving the highest priority to quoted prices (unadjusted) in active markets for identical assets and liabilities (level 1 inputs) and the lowest priority to unobservable inputs (level 3 inputs). In some cases, the inputs used to measure the fair value of an asset or a liability might be categorized within different levels of the fair value hierarchy. In those cases, the fair value measurement is categorized in its entirety in the same level of the fair value hierarchy at the lowest level input that is significant to the entire measurement. Levels used in the hierarchy are as follows:
Recognition of revenue
Revenue from contracts with customers is recognized when control of the goods or services are transferred to the customer at an amount that reflects the consideration to which the Group expects to be entitled in exchange for those goods or services. The Group has generally concluded that it is the principal in its revenue arrangements because it (i) typically controls the goods or services before transferring them to the customer, (ii) is primarily responsible for fulfilling the promise to provide the specified good or service, (iii) has inventory risk before the specified good or service has been transferred to a customer or after transfer of control to the customer, (iv) has discretion in establishing the price for the specified good or service.
The Group considers whether there are other promises in the contract that are separate performance obligations to which a portion of the transaction price needs to be allocated.
Based on the five-step model introduced in IFRS 15 - Revenue from contracts with customers, the Group recognizes revenue after the following requirements have been met:
a) the parties have approved the contract (in writing, orally or in accordance with other common commercial practices) and are committed to fulfilling the respective performance obligations;
b) the rights of each of the parties in relation to the services to be transferred can be identified;
c) the payment terms for the goods or services to be transferred can be identified;
d) the contract has commercial substance;
e) it is probable that the Group will receive the consideration to which it is entitled in exchange for the services transferred to the customer. If the consideration referred to in the contract has a variable component, the Group will estimate the amount of the consideration it will be entitled to in exchange for the services transferred to the customer.
F-15
Revenue from the sale of products and services in the Biopharmaceutical and Diagnostic Solution segment
Revenue from the sale of products in the Biopharmaceutical and Diagnostic Solution segment is mainly recognized at the point in time when control of the asset is transferred to the customer, generally on delivery of the products at the customer’s location and generally considering applicable Incoterms.
In determining the transaction price for the sale of glass and plastic products, both part of the Biopharmaceutical and Diagnostic Solution segment, the Group considers the effects of variable consideration, existence of a significant financing component, non-cash consideration, and consideration payable to the customer. If the consideration in a contract includes a variable amount, the Group estimates the amount of consideration to which it will be entitled in exchange for transferring the goods to the customer. The variable consideration is estimated at contract inception and is included in the transaction price only to the extent that it is highly probable that a significant revenue reversal in the amount of cumulative revenue recognized will not occur when the associated uncertainty with the variable consideration is subsequently resolved. The Group estimates the impact of potential returns from customers based on the Group’s right of return policies and practices along with historical data on returns, in order to determine the amount of variable consideration that can be included in the transaction price and recognized as revenue. A refund liability is recognized for the goods that are expected to be returned. Any advance payments or deposits from customers are not recognized as revenue until the control of the relevant good is transferred to the customer.
The Biopharmaceutical and Diagnostic Solution segment also develops, contracts for and sells to customers molds, tools and equipment necessary to produce plastic products. If the tooling is highly customized with no alternative use to the Group, and the Group has an enforceable right to payment for performance completed to date, revenue is recognized over time by measuring progress towards completion using the input method based on costs incurred relative to total estimated costs to completion. Otherwise, revenue for the molds, tools and equipment is recognized at the point in time when the performance obligations are satisfied by transferring of control.
The Group enters in certain contracts whereby it provides customers with the right to access certain intellectual properties for a defined short period of time. These contracts do not result in additional performance obligations for the Group and have been assessed to result in revenue to be recognized over the time the customer can benefit from the access to the intellectual property.
The normal credit term is
Revenue from the sale of products in the Engineering segment
Revenue from the sale of products in the Engineering segment is recognized at the point in time or over the time, accordingly to the terms and conditions of the customer’s contract.
The Group recognizes revenue from customer-specific construction contracts of the engineering system division over time as the performance does not create an asset with an alternative use and the Group has an enforceable right to payment for performance completed to date.
When it is not possible to consider the enforceable right to payment for performance completed to date, revenue is recognized at a point in time.
For revenue recognized over time, revenue is recognized by applying a method of measuring progress toward complete satisfaction of the related performance obligation. When selecting the method for measuring progress, the Group selects the method that best depicts the transfer of control of goods or services promised to customers. Engineering revenue is recorded under an input method, which recognizes revenue on the basis of costs incurred for the satisfaction of a performance obligation using the percentage of completion method (or expected cost plus a margin approach). The Group determines the applicable stage of completion based on the portion of contract costs incurred for work performed to date relative to the estimated total contract costs (cost to cost method).
Engineering revenue can be generated from contracts with multiple performance obligations. When a sales agreement involves multiple performance obligations, each obligation is separately identified, and the transaction price is allocated based on the amount of consideration the Group expects to be entitled in exchange for transferring the promised good or service to the customer.
F-16
There are no post-delivery obligations other than product warranties, if required by local law; these warranties do not represent a separate performance obligation and are accounted for applying IAS 37 – Provisions, Contingent Liabilities and Contingent Assets.
Engineering’s revenue also include after-sales services, which mainly consists in the supply of spare parts to customers for machinery and equipment sold, as well as maintenance activity on the machines sold. Such revenue is recognized at a point in time.
Contract costs are recognized in profit or loss as incurred unless they create an asset which mainly generates or enhances resources that will be used in satisfying (or in continuing to satisfy) performance obligations in the future. When it is probable that total contract costs will exceed total contract revenue, the expected loss is recognized as an expense immediately in the consolidated income statement following requirements on onerous contracts in IAS 37.
Costs to obtain a contract
According to IFRS 15 the Group recognizes incremental costs of obtaining a contract as an asset if the required criteria are met. Any capitalized contract costs asset is amortized on a systematic basis that is consistent with the entity’s transfer of the related goods or services to the customer.
The Group presents these costs in the statement of financial position as a separate class of intangible asset, with the amortization in the same line item as amortization of intangible assets within the scope of IAS 38 - Intangible Assets.
Capitalized contract costs are subject to an impairment assessment at the end of each reporting period. Impairment losses are recognized in profit or loss.
Government grants
Government grants are recognized when there is reasonable assurance that the grant will be received and all attached conditions will be complied with. When the grant relates to an expense item, it is recognized as income on a systematic basis over the periods that the related costs, for which it is intended to compensate, are expensed. When the grant relates to an asset, it is recognized as income in equal amounts over the expected useful life of the related asset.
The Group has elected to present government grants in the statement of financial position as other liabilities, which is recognized in the income statement among other operating income on a systematic and rational basis over the useful life of the asset they relate to.
The Group has chosen to present government grants related to an expense item as other operating income in the income statement or netted within the related costs according to the nature of the contribution.
Trade receivables
A receivable is the entity’s right to consideration that is unconditional. A right to consideration is unconditional only if the passage of time is required before payment of that consideration is due.
Contract assets
The entity’s right to consideration in exchange for goods or services that the entity has transferred to a customer when that right is conditioned on something other than the passage of time.
Contract liabilities
A contract liability is the entity’s obligation to transfer goods or services to a customer for which the entity has received consideration.
Presentation of Contract assets and liabilities
Contract assets and liabilities are determined at the contract level and not at the performance obligation level. As such, an asset or liability for each performance obligation within a contract is not separately recognized, but they are aggregated into a single contract asset or liability. Contract asset or contract liability positions are determined for each contract on a net basis.
F-17
Cost of sales
Cost of sales comprises expenses incurred in the manufacturing and distribution of products. The remaining costs principally include depreciation, amortization directly related to the manufacturing process and transportation costs.
Transaction costs for
In accordance with IAS 32 - Financial instrument: presentation, the transaction costs of an equity transaction are accounted for as a deduction from equity to the extent they are incremental costs directly attributable to the equity transaction that otherwise would have been avoided. Transaction costs are mainly related to underwriting commissions and consultancy costs. Transaction costs relate jointly to offering of share and stock exchange listing of new shares have been allocated to those transactions using a basis of allocation that is rational, based on the proportion of primary and secondary offering of shares.
Income (and deferred) taxes
Income taxes include all the taxes calculated on taxable profits of the Group. Income taxes are recorded in the income statement, except to the extent that they relate to a business combination, or items recognized directly in equity or in other comprehensive income.
Current taxes are calculated on the basis of the tax laws enacted or substantially enacted at the reporting date in the countries where the Group operates and generates taxable income. Current tax receivables and payables are measured at the amount expected to be recovered or paid to the tax authorities.
The Italian Regional Income Tax (“IRAP”) is recognized within income tax expense. IRAP is calculated on a measure of income defined by the Italian Civil Code as the difference between operating revenue and costs, before financial income and expense, and in particular before the cost of fixed-term employees, credit losses and any interest included in lease payments, for the Italian components of the Group only. IRAP is applied on the tax base at
Deferred tax is provided using the liability method on temporary differences between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes at the reporting date.
Deferred tax liabilities are recognized for all taxable temporary differences, except when the deferred tax liability arises from the initial recognition of goodwill or an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither the accounting profit nor taxable profit or loss unless it gives rise to equal taxable and deductible temporary differences.
Deferred tax assets are recognized for all deductible temporary differences, the carry forward of unused tax credits and any unused tax losses. Deferred tax assets are recognized to the extent that it is probable that taxable profit will be available against which the deductible temporary differences, and the carry forward of unused tax credits and unused tax losses can be utilized, unless the deferred tax assets arise from the initial recognition of an asset or liability in a transaction that is not a business combination and at the time of the transaction, affects neither accounting profit nor taxable profit.
The carrying amount of deferred tax assets is reviewed at each reporting date and reduced to the extent that it is no longer probable that sufficient taxable profit will be available to allow all or part of the deferred tax asset to be utilized. Unrecognized deferred tax assets are re-assessed at each reporting date and are recognized to the extent that it has become probable that future taxable profits will allow the deferred tax asset to be recovered.
Deferred tax assets and liabilities are measured at the tax rates that are expected to apply to the period when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted at the reporting date.
In assessing the feasibility of the realization of deferred tax assets, management considers whether it is probable that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible and the tax loss carry-forwards are utilized. Estimating future taxable income requires estimates about matters that are inherently uncertain and requires significant management judgment, and different estimates can have a significant impact on the outcome of the analysis.
F-18
Changes in the assumptions and estimates related to future taxable income, tax planning strategies and scheduled reversal of deferred tax liabilities could affect the recoverability of the deferred tax assets. If actual results differ from such estimates and assumptions, the Group financial position and results of operation may be affected.
Deferred tax relating to items recognized outside profit or loss is recognized outside profit or loss. Deferred tax items are recognized in correlation to the underlying transaction either in OCI or directly in equity. The Group offsets deferred tax assets and deferred tax liabilities if and only if it has a legally enforceable right to set off current tax assets and current tax liabilities and the deferred tax assets and deferred tax liabilities relate to income taxes levied by the same taxation authority on either the same taxable entity or different taxable entities which intend either to settle current tax liabilities and assets on a net basis, or to realize the assets and settle the liabilities simultaneously, in each future period in which significant amounts of deferred tax liabilities or assets are expected to be settled or recovered.
Any uncertainty regarding tax treatments is considered in the tax calculation in accordance with the requirements in IFRIC 23 - Uncertainty over Income Tax Treatments whereby an entity considers whether it is probable that a taxation authority will accept an uncertain tax treatment. If the Group concludes that the position is not probable of being accepted, the effect of uncertainty is reflected in the income taxes.
Dividends
The Company recognizes a liability to pay dividends when the distribution is authorized and the distribution is no longer at the discretion of the Company. As per the corporate laws of Italy, a distribution is authorized when it is approved by the shareholders. A corresponding amount is recognized as a decrease in equity.
Other intangible assets
Intangible assets, other than goodwill, acquired separately are measured on initial recognition at cost. The cost of intangible assets acquired in a business combination is their fair value at the date of acquisition. Following initial recognition, intangible assets are carried at cost less any accumulated amortization and accumulated impairment losses. Internally generated intangibles, excluding capitalized development costs, are not capitalized and the related expenditure is reflected in profit or loss in the period in which the expenditure is incurred. The useful lives of intangible assets are assessed as either finite or indefinite. Intangible assets with finite lives are amortized over the useful economic life and assessed for impairment whenever there is an indication that the intangible asset may be impaired. The amortization period and method for an intangible asset with a finite useful life are reviewed at the end of each reporting period. Changes in the expected useful life or the expected pattern of consumption of future economic benefits embodied in the asset are considered to modify the amortization period or method and are treated as changes in accounting estimates. The amortization expense on intangible assets with finite lives is recognized in the income statement in the expense category that is consistent with the function of the intangible assets.
Developments costs for the production of new products or parts, as requested by IAS 38 - Intangible Assets, are recognized as assets only if (i) the costs can be reliably determined, (ii) the Group has the intention and resources to complete them, the technical feasibility of completing them is such that they will be available for use, (iii) the Group has the intention to complete and the ability and intention to use or sell the asset, (iv) the asset will generate future economic benefits, (v) there are availability of resources to complete the asset and the ability to measure reliably the expenditure during development. Capitalized development costs include only those expenses that can be directly attributed to the development process and are amortized on a systematic basis, starting from the commencement of production and lasting the length of the product or process's estimated life, generally ranging between and
Industrial patents and intellectual property rights, and licenses are valued at purchase or production cost and amortized, if they have a finite life, on a straight-line basis over their estimated useful life, generally between and
Other intangible assets mainly relate to the registration of trademarks and have been recognized in accordance with IAS 38 - Intangible Assets, where it is probable that the use of the asset will generate future economic benefits for the Group and where the cost of the asset can be measured reliably. Other intangible assets are measured at cost less any impairment losses and amortized on a straight-line basis over their estimated life, which is generally between and
An intangible asset is derecognized upon disposal (i.e., at the date the recipient obtains control) or when no future economic benefits are expected from its use or disposal. Any gain or loss arising upon derecognition of the asset (calculated as the difference between the net disposal proceeds and the carrying amount of the asset) is included in the income statement.
F-19
Property, plant and equipment
Plant and equipment are recorded at purchase or production cost and systematically depreciated over their residual useful lives and accumulated impairment losses, if any. Such cost includes the cost of replacing part of the plant and equipment and borrowing costs for long-term construction projects if the recognition criteria are met.
When significant parts of plant and equipment are required to be replaced at intervals, the Group depreciates them separately based on their specific useful lives. Likewise, when a major inspection is performed, its cost is recognized in the carrying amount of the plant and equipment as a replacement if the recognition criteria are satisfied. All other repair and maintenance costs are recognized in profit or loss as incurred. The present value of the expected cost for the decommissioning of an asset after its use is included in the cost of the respective asset if the recognition criteria for a provision are met. Construction in progress is stated at cost, net of accumulated impairment losses, if any.
The useful lives, estimated by the Group for its various categories of property, plant and equipment, are as follows:
|
|
Biopharmaceutical |
|
Engineering |
|
Holding |
Buildings |
|
|
|
|||
Plant and machinery |
|
|
|
|||
Industrial and commercial equipment |
|
|
|
|||
Other tangible assets |
|
|
|
Land is not depreciated. The residual values, useful lives and methods of depreciation of property, plant and equipment are reviewed at each financial year end and adjusted prospectively, if appropriate.
An item of property, plant and equipment and any significant part initially recognized is derecognized upon disposal (i.e., at the date the recipient obtains control) or when no future economic benefits are expected from its use or disposal. Any gain or loss arising on derecognition of the asset (calculated as the difference between the net disposal proceeds and the carrying amount of the asset) is included in the income statement when the asset is derecognized.
Borrowing costs
General and specific borrowing costs directly attributable to the acquisition, construction or production of qualifying assets, which are assets that necessarily take a substantial period of time to get ready for their intended use, are added to the cost of those assets, until such time as the assets are substantially ready for their intended use.
All other borrowing costs are expensed in net financial expenses in the consolidated income statement, as incurred.
Leases
The Group assesses at contract inception whether a contract is, or contains, a lease. That is, if the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration.
According to IFRS 16 - Leases, the Group applies a recognition and measurement approach for each lease, except for short-term leases (i.e., those leases that have a lease term of 12 months or less) and leases of low-value assets (i.e., leases of underlying assets with a value, when new, of EUR
The Group recognizes lease liabilities representing obligations to make lease payments and right of use assets representing the right of use the underlying assets.
The Group recognizes right of use assets at the commencement date of the lease and it is measured at cost, less any accumulated depreciation and impairment losses, and adjusted for any remeasurement of lease liabilities. Right of use assets are measured at cost comprising the following: (i) the amount of the initial measurement of lease liability; (ii) any lease payments made at or before the commencement date less any lease incentives received; (iii) any initial direct costs and, if applicable, (iv) restoration costs. Right of use assets are depreciated on a straight-line basis over the shorter of the lease term and the estimated useful lives of the assets.
F-20
At the commencement date of the lease, the Group recognizes lease liabilities measured at the present value of lease payments to be made over the lease term, of the following: (i) fixed lease payments less any lease incentives receivable, (ii) variable lease payments that are based on an index or a rate and, if applicable, (iii) amounts expected to be payable under residual value guarantees, and (iv) the exercise price of a purchase option if the lessee is reasonably certain to exercise that option. Lease payments are discounted using the interest rate implicit in the lease. If that rate cannot be determined, the Group’s incremental borrowing rate is used, being the rate that the Group would have to pay to borrow the funds necessary to obtain an asset of similar value in a similar economic environment with similar terms and conditions. Each lease payment is allocated between the principal liability and interest expense. Interest expense is charged to the income statement over the lease period using the effective interest rate method.
Inventories
Inventories of raw materials, semi-finished and finished products are valued at the lower of cost and net realizable value. Costs is determined, as follows:
Allowances for obsolete and slow-moving goods are calculated for materials and finished products, taking into account their future expected use and realizable value. Net realizable value is the estimated selling price in the ordinary course of business, less estimated costs of completion and the estimated costs necessary to make the sale.
Financial instruments
A financial instrument is a contract that gives rise to a financial asset of one entity and a financial liability or equity instrument of another entity. Current financial assets include trade receivables, derivative financial instruments, other current financial assets and cash and cash equivalents. Investments and other financial assets include investments accounted for using the equity method, as well as other securities, derivative financial instruments and non-current financial assets. Financial liabilities include debt and borrowings from banks, trade payables and other financial liabilities, which mainly include derivative financial instruments.
Financial assets
Financial assets are classified on the basis of the impairment model introduced by IFRS 9 – Financial instruments, at initial recognition, as subsequently measured at amortized cost, fair value through other comprehensive income (OCI), and fair value through profit or loss.
The Group initially measures a financial asset at its fair value plus transaction costs, in the case of a financial asset not at fair value through profit or loss.
Trade receivables are stated at amortized cost and are measured on the basis of the impairment model introduced by IFRS 9. In accordance with this model, the Group recognizes a loss allowance based on lifetime ECLs (Expected Credit Losses) at each reporting date, based on its historical credit loss experience, adjusted for forward-looking factors specific to the debtors and the economic environment. The amount of receivables is reported in the statement of financial position net of the relevant credit loss provisions. The impairment losses reported pursuant to IFRS 9 (including reversals of impairment losses or impairment gains) are recognized in the consolidated income statement within the line item Selling and marketing expenses.
Financial assets are derecognized when the rights to receive cash flows from the instrument have expired and the Group has transferred substantially all risks and rewards of ownership.
Financial assets at fair value through profit or loss (FVTPL) are carried in the statement of financial position at fair value with net changes in fair value recognized in the income statement. This category includes derivative instruments and equity investments which the Group has not irrevocably elected to classify at fair value through OCI.
As permitted by IFRS 9, equity investments for which there is no quoted market price in an active market and there is insufficient financial information in order to determine fair value may be measured at cost as an estimate of fair value.
F-21
Financial liabilities
Financial liabilities are classified, at initial recognition, as financial liabilities at fair value through profit or loss, loans and borrowings, payables, or as derivatives designated as hedging instruments in an effective hedge, as appropriate.
All financial liabilities are recognized initially at fair value and, in the case of loans and borrowings and payables, net of directly attributable transaction costs. The Group’s financial liabilities include trade and other payables, loans and borrowings including bank overdrafts, and derivative financial instruments.
For purposes of subsequent measurement, financial liabilities are classified as financial liabilities at fair value through profit or loss or financial liabilities at amortized cost (loans and borrowings).
Financial liabilities at fair value through profit or loss include financial liabilities held for trading and financial liabilities designated upon initial recognition as at fair value through profit or loss. The Group has not designated any financial liability as at fair value through profit or loss.
Financial liabilities at amortized cost is the category most relevant to the Group. After initial recognition, interest-bearing loans and borrowings are subsequently measured at amortized cost using the EIR (Effective Interest Rate) method. Gains and losses are recognized in profit or loss when the liabilities are derecognized as well as through the EIR amortization process.
Amortized cost is calculated by considering any discount or premium on acquisition and fees or costs that are an integral part of the EIR. The EIR amortization is included as interest expense in the income statement.
A financial liability is derecognized when the obligation under the liability is discharged or canceled or expires.
Borrowings are classified among current liabilities, unless the Group has an unconditional right to defer their payment for at least twelve months after the reporting date.
Derivatives
Derivative financial instruments are accounted for in accordance with IFRS 9. At the inception of the contract, derivative instruments are initially recognized at fair value as financial assets at FVTPL when the fair value is positive, or financial liabilities at FVTPL when the fair value is negative.
When a derivative financial instrument is designated as a hedge of the exposure to variability in future cash flows or highly probable forecasted transactions, the effective portion of the gain or loss on the hedging instrument is recognized in OCI in the cash flow hedge reserve, while any ineffective portion is recognized immediately in the income statement. The Group uses IRS contract (Interest Rate Swap) and commodity swaps as hedges of its exposure to financial interest of loans and to commodity price risks respectively. The cash flow hedge reserve is adjusted to the lower of the cumulative gain or loss on the hedging instrument and the cumulative change in fair value of the hedged item.
The Group uses forward currency and collar contracts as hedges of its exposure to foreign currency risk in forecast transactions and firm commitments, for its exposure to volatility of exchange rates. The ineffective portion is recognized in financial income or expenses.
The Group designates only the spot element of forward contracts as a hedging instrument, forward points are formally excluded from the hedging relationship and accounted as cost of hedging. The forward element is recognized in OCI and accumulated in a separate component of equity under Cost of Hedging Reserve.
Impairment of non-financial assets
If any indication exists, or when annual impairment testing for an asset is required, the Group estimates the asset’s recoverable amount. An asset’s recoverable amount is the higher of an asset’s or CGU’s fair value less costs of disposal and its value in use. If it is not possible to estimate the recoverable amount of an individual asset, the Group assesses whether the cash-generating unit to which it belongs is impaired. When the carrying amount of an asset or CGU exceeds its recoverable amount, the asset is considered impaired and is written down to its recoverable amount. For assets excluding goodwill, whether there is an indication that previously recognized impairment losses no longer exist or have decreased, the Group estimates the asset’s or CGU’s recoverable amount. A previously recognized impairment loss is reversed only if there has
F-22
been a change in the assumptions used to determine the asset’s recoverable amount since the last impairment loss was recognized.
Cash and cash equivalents
Cash and cash equivalents in the statement of financial position comprise cash on hand and at bank, carried at nominal amount, equal to fair value. Cash equivalents are short-term (i.e. with a maturity period of 90 days or less), highly liquid investments that are readily convertible to known amounts of cash and which are subject to an insignificant risk of changes in value.
Equity
Retained earnings and other reserves include undistributed earnings of the Group, the accumulated amount of items recognized in other comprehensive income (such as actuarial gains and losses, cash-flow hedge reserves, etc.) and other reserves (translation differences). Dividends are deducted from equity when they are approved by the Shareholders’ Meeting.
Non-controlling interests represent the portion of the net assets and net profit of a consolidated entity that is not attributable to the Group, directly or indirectly.
Provisions
Provisions for risks are recognized when (i) the Group has a present obligation, legal or constructive, as a result of a past event; (ii) it is probable that the outflow of resources will be required; (iii) the amount of the obligation can be reliably estimated. Provisions are determined by the Group based on facts and circumstances, historical risk data and the information available at the balance sheet date. When the Group expects some or all of a provision to be reimbursed, for example, under an insurance contract, the reimbursement is recognized as a separate asset, but only when the reimbursement is virtually certain. Where the effect of the time value of money is material and the date of extinguishing the liability can be reasonably estimated, provisions are stated at the present value of the expected expenditure, using a discount rate that reflects current market assessments of the time value of money and the risks specific to the liability. When discounting is used, the increase in the provision due to the passage of time is recognized as an interest expense. Contingencies for which the probability of a liability is remote are disclosed in the notes, but no provision is recognized.
Employee benefits
Employee severance indemnity, mandatory for Italian companies pursuant to Article 2120 of the Italian Civil Code, is deferred compensation and is based on the employees' years of service and the compensation earned by the employee during the service period. Under IAS 19 - Employee Benefits, the employee severance indemnity as calculated is considered a "Defined benefit plan" and the related liability recognized in the statement of financial position (Employees benefits) is determined by actuarial calculations.
The remeasurements of actuarial gains and losses are recognized in other components of the Consolidated statements of comprehensive income.
Starting from January 1, 2007, Italian Law gave employees the choice to direct their accruing indemnity either to supplementary pension funds or leave the indemnity as an obligation of the Company. Companies that employ at least 50 employees should transfer the employee severance indemnity to the "Treasury fund" managed by INPS, the Italian Social Security Institute. Consequently, the Group's obligation to INPS and the contributions to supplementary pension funds take the form, under IAS 19, of a "Defined contribution plan".
Net interest is calculated by applying the discount rate to the net defined benefit liability or asset. The Group recognizes the following changes in the net defined benefit obligation under expenses in the consolidated statement of profit or loss:
F-23
Share-based payments
Employees (including senior executives) of the Group receive remuneration in the form of share-based payments, whereby employees render services in exchange for equity instruments (equity-settled transactions). The share-based compensation arrangements are accounted for in accordance with IFRS 2 - Share-based Payment, which requires the Company to recognize share-based compensation expense based on fair value of awards granted.
The cost of equity-settled transactions is determined by the fair value at the date when the grant is made. Service and non-market performance conditions are not taken into account when determining the grant date fair value of awards, but the likelihood of the conditions being met is assessed as part of the Company’s best estimate of the number of equity instruments that will ultimately vest. Market performance conditions are reflected within the grant date fair value.
The fair value of the awards which are conditional only on a recipient’s continued service to the Company is measured using the share price at the grant date adjusted for the present value of future distributions which employees will not receive during the vesting period.
That cost is recognized in employee benefits expense, together with a corresponding increase in a reserve of shareholder’s equity, over the period in which the service and, where applicable, the performance conditions are fulfilled (the vesting period). The cumulative expense recognized for equity-settled transactions at each reporting date until the vesting date reflects the extent to which the vesting period has expired and the Group’s best estimate of the number of equity instruments that will ultimately vest. The expense or credit in the income statement for a period represents the movement in cumulative expense recognized as at the beginning and end of that period.
At each reporting date, the Group revises the assumptions about the number of shares expected to be accrued and recognizes the effect of any change in the estimate to the income statement, adjusting the corresponding equity reserve.
Trade payables
Trade payables are obligations to pay for goods or services that have been acquired in the ordinary course of business from suppliers. Trade payables are classified as current liabilities if payment is due within one year or less from the reporting date. If not, they are presented as non-current liabilities. Trade payables are initially recognized at fair value and subsequently measured at amortized cost.
Other current and non-current liabilities
The Group recognizes liabilities from other taxes and social security and other non-financial liabilities at amount payable on the maturity date. Pre-payments received on orders as well as the liability balance from constructions contracts are reported as contract liabilities.
Significant judgements and estimates
The consolidated financial statements are prepared in accordance with IFRS which require management’s use of judgments, estimates and assumptions that may affect the carrying amount of assets, liabilities, income and expenses in the financial statements, as well as the disclosures in the notes concerning contingent assets and liabilities at the balance sheet date.
Uncertainty surrounding these assumptions and estimates might lead to results that require a material adjustment to carrying amounts of assets or liabilities in future periods.
Estimates are based on historical experience and other factors. The resulting accounting estimates could differ from the related actual results. Estimates are periodically reviewed and the effects of each change are reflected in the consolidated income statement or in the consolidated statement of comprehensive income in the period in which the change occurs.
In preparing the consolidated financial statements, management has considered the impact of climate change in the context of the disclosures. These considerations did not have a material impact on the financial reporting judgements and
F-24
estimates, consistent with the assessment that climate change is not expected to have a significant impact on the Group’s going concern assessment, its ability to recover the carrying value of its long-lived assets or its liquidity.
Key sources of estimation uncertainty
Revenue Recognition
The Group operates in several jurisdictions and assesses whether contracts with customers provide it with the right to consideration for the performance fulfilled based on legal assessment of applicable contracts and other source of enforceable rights and obligations (i.e. local regulations). As regards revenue from contracts with customers for contract work and contract assets and liabilities, application of the cost-to-cost method requires a prior estimate of the entire lifetime costs of individual projects, updating them at each balance sheet date. This requires assumptions, those can be affected by multiple factors, such as the time over which some projects are developed, their high level of technology and innovative content, the possible presence of price variations and revisions, and machinery performance guarantees, including an estimate of contractual risks, where applicable. These facts and circumstances make it difficult to estimate the projects' costs to complete and, consequently, to estimate the value of contract work in progress at the balance sheet date. The Group estimates variable considerations to be included in the transaction price for the sale of products with rights of return and volume rebates. The Group forecasts sales returns using the historical return data to come up with expected return percentages. These percentages are applied to determine the expected value of the variable consideration.
Recoverable amount of goodwill
The impairment test on goodwill is carried out by comparing the carrying amount of cash-generating units on which it is allocated and their recoverable amount. The recoverable amount of a cash-generating unit is the higher of fair value, less costs to sell, and its value in use. The value in use is determined using the discounted cash flow method which uses assumptions to estimate cash flows. The recoverable amount depends significantly on the discount rate used in the discounted cash flow model as well as the expected future cash flows and the growth rate used for the extrapolation. The key assumptions used to determine the recoverable amount for the different cash-generating units, including a sensitivity analysis, are detailed in the Note 16. For the years ended December 31, 2023, 2022 and 2021
Use of estimates
Employee benefit liabilities
Employee benefit liabilities: employee benefits, especially the provision for employee severance indemnities and other long term incentives, are calculated using actuarial assumptions; changes in such assumptions could have a material impact on such liabilities.
Leases
The Group cannot readily determine the interest rate implicit in the lease, therefore, it uses its incremental borrowing rate (IBR) to measure lease liabilities. The IBR is the rate of interest that the Group would have to pay to borrow over a similar term, and with a similar security, the funds necessary to obtain an asset of a similar value to the right-of-use asset in a similar economic environment. The IBR therefore reflects what the Group ‘would have to pay’, which requires estimation when no observable rates are available (such as for subsidiaries that do not enter into financing transactions) or when they need to be adjusted to reflect the terms and conditions of the lease (for example, when leases are not in the subsidiary’s functional currency). The Group estimates the IBR using observable inputs (such as market interest rates) when available and is required to make certain entity-specific estimates (such as the subsidiary’s stand-alone credit rating). The Group determines the lease term as the non-cancellable term of the lease, together with any periods covered by an option to extend the lease if it is reasonably certain to be exercised, or any periods covered by an option to terminate the lease, if it is reasonably certain not to be exercised. The Group applies judgement in evaluating whether it is reasonably certain whether or not to exercise the option to renew or terminate the lease. That is, it considers all relevant factors that create an economic incentive for it to exercise either the renewal or termination.
Expected credit losses of trade receivables and contract assets
The Group uses a simplified approach in calculating ECLs for trade receivables and contract assets, initially based on the Group’s historical observed default rates. The Group will adjust the historical credit loss experience with forward-looking information. At every reporting date, the historical observed default rates are updated and changes in the forward-looking
F-25
estimates are analyzed. The assessment of the correlation between historical observed default rates, forecast economic conditions and ECLs is a estimate. The amount of ECLs is sensitive to changes in circumstances and of forecast economic conditions. The Group’s historical credit loss experience and forecast of economic conditions may also not be representative of customer’s actual default in the future.
Income tax expense (current and deferred)
The Group is subject to different tax jurisdictions. The determination of tax liabilities for the Group requires the use of assumptions with respect to transactions whose fiscal consequences are not yet certain at the end of the reporting period. Calculation of taxes on a global scale requires the use of estimates and assumptions based on the information available at the balance sheet date. The deferred tax assets realization is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible and the tax loss carried forwards can be utilized. Estimating future taxable income requires estimates about matters that are inherently uncertain and requires significant management judgment, and different estimates can have a significant impact on the outcome of the analysis.
Share-based compensation
The Group accounts for its equity incentive plan in accordance with IFRS 2 - Share-based Payment, which requires the recognition of share-based compensation expense based on the fair value of the awards granted. Share-based compensation for equity-settled awards requires the input of subjective assumptions, including the dividend yield, the probability that the Group will achieve the performance target and the hypotheses of the beneficiaries leaving. As a result, at the grant date management is required to make key assumptions and estimates regarding conditions that will occur in the future, which inherently involves uncertainty. Therefore, the amount of share-based compensation recognized has been affected by the significant assumptions and estimates used.
3. Changes in accounting policies and disclosures
New accounting standards
The principles and standards utilized in preparing these consolidated financial statements have been consistently applied through all periods presented, with the exception of the new standards and interpretations that are effective for reporting periods beginning on January 1, 2023, described below.
New standards and amendments effective from January 1, 2023
The following new standards and amendments effective from January 1, 2023 were adopted by the Group for the preparation of these Consolidated Financial Statements.
In May 2017, the IASB issued IFRS 17 — Insurance Contracts, which establishes principles for the recognition, measurement, presentation and disclosure of insurance contracts issued as well as guidance relating to reinsurance contracts held and investment contracts with discretionary participation features issued. In June 2020 the IASB issued amendments to IFRS 17 aimed at helping companies implement IFRS 17 and make it easier for companies to explain their financial performance. The new standard and amendments are effective on or after January 1, 2023. There was no effect from the adoption of these amendments.
In February 2021, the IASB issued amendments to IAS 1 — Presentation of Financial Statements and IFRS Practice Statement 2: Disclosure of Accounting Policies which require companies to disclose their material accounting policy information rather than their significant accounting policies and provide guidance on how to apply the concept of materiality to accounting policy disclosures. These amendments are effective on or after January 1, 2023. Certain accounting policy disclosures were updated a result of the adoption of these amendments.
In February 2021, the IASB issued amendments to IAS 8 — Accounting Policies, Changes in Accounting Estimates and Errors: Definition of Accounting Estimates which clarify how companies should distinguish changes in accounting policies from changes in accounting estimates. These amendments are effective on or after January 1, 2023. There was no effect from the adoption of these amendments.
In May 2021, the IASB issued amendments to IAS 12 — Income Taxes: Deferred Tax related to Assets and Liabilities Arising From a Single Transaction that clarify how companies account for deferred tax on transactions such as leases and
F-26
decommissioning obligations. These amendments are effective on or after January 1, 2023. There was no effect from the adoption of these amendments
In December 2021, the IASB issued amendments to IFRS 17 — Insurance Contracts: Initial Application of IFRS 17 and IFRS 9 - Comparative Information, which provides a transition option relating to comparative information about financial assets presented on initial application of IFRS 17. The amendment is aimed at helping entities to avoid temporary accounting mismatches between financial assets and insurance contract liabilities, and therefore improve the usefulness of comparative information for users of financial statements. The amendment is effective on or after January 1, 2023. There was no effect from the adoption of these amendments.
In June 2020, the IASB issued amendments to IFRS 4 — Insurance Contracts which defer the expiry date of the temporary exemption from applying IFRS 9 to annual periods beginning on or after January 1, 2023. There was no effect from the adoption of these amendments.
In May 2023, the IASB issued amendments to IAS 12 — Income taxes: International Tax Reform – Pillar Two Model Rules, to clarify the application of IAS 12 — Income taxes to income taxes arising from tax law enacted or substantively enacted to implement the Organisation for Economic Co-operation and Development (OECD)/G20 Inclusive Framework on Base Erosion and Profit Shifting (BEPS) Pillar Two model rules (Pillar Two income taxes). The amendments introduce: (i) a mandatory temporary exception to the accounting for deferred taxes arising from the jurisdictional implementation of the Pillar Two model rules, which was effective immediately upon issuance of the amendment, and (ii) disclosure requirements for affected entities to help users of the financial statements better understand an entity’s exposure to Pillar Two income taxes arising from that legislation, particularly before the effective date of the Pillar Two model rules, which apply for annual reporting periods beginning on or after January 1, 2023, but not for any interim periods ending on or before December 31, 2023. The Group started applying the mandatory temporary exception to accounting for deferred taxes arising from the Pillar Two model rules on its effective date.
The Pillar Two model rules introduce a minimum effective taxation of 15 percent on a jurisdictional basis for multinational enterprise groups and large-scale domestic groups with annual revenues of at least €750 million in their consolidated financial statements in at least two of the four prior fiscal years. Many countries where the Group operates have enacted domestic tax legislation for the Pillar Two model rules that are effective from January 1, 2024, including Italy. The Group did not recognize any tax expense or liability relating to Pillar Two in 2023 as the legislation was not in effect at the reporting date. The Pillar Two model rules are complex and management is in the process of assessing and determining its impact on the Group.
New standards, amendments and interpretations not yet effective
The standards, amendments and interpretations issued by the IASB that will have mandatory application in 2024 or subsequent years are listed below:
In January 2020, the IASB issued amendments to IAS 1 — Presentation of Financial Statements: Classification of Liabilities as Current or Non-Current to clarify how to classify debt and other liabilities as current or non-current, and in particular how to classify liabilities with an uncertain settlement date and liabilities that may be settled by converting to equity. These amendments are effective on or after January 1, 2024. The Group does not expect any material impact from the adoption of these amendments.
In September 2022, the IASB issued amendments to IFRS 16 — Leases: Liability in a Sale and Leaseback to improve the requirements for sale and leaseback transactions, which specify the measurement of the liability arising in a sale and leaseback transaction, to ensure the seller-lessee does not recognize any amount of the gain or loss that relates to the right of use it retains. These amendments are effective on or after January 1, 2024. The Group does not expect any material impact from the adoption of these amendments.
In October 2022, the IASB issued amendments to IAS 1 — Presentation of Financial Statements: Non-current Liabilities with Covenants, that clarify how conditions with which an entity must comply within twelve months after the reporting period affect the classification of a liability. These amendments are effective on or after January 1, 2024. The Group does not expect any material impact from the adoption of these amendments.
In May 2023, the IASB issued amendments to IAS 7 — Statement of Cash Flows and IFRS 7 — Financial Instruments: Disclosures: Supplier Finance Arrangements, that introduce new disclosure requirements to enhance the transparency and usefulness of the information provided by entities about supplier finance arrangements and are intended to assist users of
F-27
financial statements in understanding the effects of supplier finance arrangements on an entity’s liabilities, cash flows and exposure to liquidity risk. The amendments are effective on or after January 1, 2024. The Group does not expect any material impact from the adoption of these amendments.
In August 2023, the IASB issued amendments to IAS 21 — The Effects of Changes in Foreign Exchange Rates: Lack of Exchangeability, to clarify how an entity has to apply a consistent approach to assessing whether a currency is exchangeable into another currency and, when it is not, to determine the exchange rate to use and the disclosures to provide. These amendments are effective on or after January 1, 2025. The Group does not expect any material impact from the adoption of these amendments.
4. Scope of consolidation
Stevanato Group S.p.A. is the parent company of the Group and it holds, directly and indirectly, interests in the Group’s operating companies. The Group’s scope of consolidation at December 31, 2023 and 2022 is as follows:
Subsidiaries
The consolidated financial statements of the Group include the following list of companies directly or indirectly through the subsidiaries Stevanato Group International a.s., Balda Medical GmbH, Spami S.r.l., controlled by the parent company Stevanato Group S.p.A.:
|
|
|
|
|
|
|
|
% equity interest |
|
|||
Name |
|
Segment |
|
Description |
|
Country of incorporation |
|
2023 |
|
2022 |
|
|
Nuova Ompi S.r.l. |
|
|
|
|
|
|
||||||
Spami S.r.l. |
|
|
|
|
|
|
||||||
Stevanato Group International a.s. |
|
|
|
|
|
|
||||||
Medical Glass a.s. |
|
|
|
|
|
|
||||||
Stevanato Group N.A. S. de RL de CV |
|
|
|
|
|
|
||||||
Ompi N.A. S. de RL de CV |
|
|
|
|
|
|
||||||
Ompi of America inc. |
|
|
|
|
|
|
||||||
Ompi do Brasil I. e C. de Em. Far. Ltda |
|
|
|
|
|
|
||||||
Perugini S.r.l. |
|
|
|
|
|
|
— |
|
||||
Ompi Pharm. Packing Techn. Co. Ltd |
|
|
|
|
|
|
||||||
Stevanato Group Denmark A/S (*) |
|
|
|
|
|
|
||||||
Medirio SA |
|
|
|
|
|
|
||||||
Balda Medical Gmbh |
|
|
|
|
|
|
||||||
Balda C. Brewer Inc. |
|
|
|
|
|
|
||||||
Balda Precision Inc. |
|
|
|
|
|
|
||||||
Ompi of Japan Co., Ltd. |
|
|
|
|
|
|
||||||
F-28
(*) On December 31, 2022, the respective extraordinary shareholders' meetings of Innoscan A/S and SVM Automatik A/S approved the merger of Innoscan A/S into SVM Automatik A/S. The transaction was effective for accounting purposes at January 1, 2022. In February 2023, the surviving company SVM Automatik A/S changed its corporate name to Stevanato Group Denmark A/S.
Change in scope of consolidation - business combination
On November 8, 2023, the subsidiary Spami S.r.l. acquired all of the business operations of Perugini S.r.l., an Italian company specialized in the manufacturing of consumables and mechanical components for industrial machines. The acquisition of Perugini will support the Group's efforts in the ongoing integration of critical technologies and processes into the Group’s production process.
As the assets acquired and the liabilities assumed constitute the acquisition of a business, the transaction is considered a business combination pursuant to IFRS 3.
The initial net consideration was paid entirely at the closing date based on the pro-forma financial statements of Perugini S.r.l. The consideration would have been adjusted based on the definitive net financial position at the closing date, as defined in the share purchase agreement. The consideration adjustment was to be agreed among parties within
Details of the purchase consideration are as follows:
|
|
At acquisition date |
|
|
|
|
(EUR thousand) |
|
|
Consideration paid |
|
|
|
|
Consideration adjustment |
|
|
|
|
Total consideration |
|
|
|
|
F-29
Consequently, the consideration was temporarily allocated to the assets and liabilities acquired while the definitive purchase price allocation will be made within twelve months of the acquisition. Details of the net assets acquired and goodwill are as follows:
|
|
Preliminary fair value at the acquisition date |
|
|
|
|
(EUR thousand) |
|
|
Right of use assets |
|
|
|
|
Property, plant and equipment |
|
|
|
|
Deferred tax assets |
|
|
|
|
Inventories |
|
|
|
|
Trade receivables |
|
|
|
|
Other receivables |
|
|
|
|
Cash and cash equivalents |
|
|
|
|
Total assets |
|
|
|
|
Non-current financial liabilities |
|
|
|
|
Employee benefits |
|
|
|
|
Deferred tax liabilities |
|
|
|
|
Other non-current liabilities |
|
|
|
|
Current financial liabilities |
|
|
|
|
Trade payables |
|
|
|
|
Tax payables |
|
|
|
|
Other current liabilities |
|
|
|
|
Total liabilities |
|
|
|
|
|
|
|
|
|
Net assets |
|
|
|
|
|
|
|
|
|
Portion acquired by the Group ( |
|
|
|
|
|
|
|
|
|
Goodwill |
|
|
|
|
Minor acquisition-related costs were expensed and recorded within selling and marketing costs in the consolidated income statement.
The details of the net cash outflows related to the acquisition are shown below:
|
|
At acquisition date |
|
|
|
|
(EUR thousand) |
|
|
Consideration paid |
|
|
( |
) |
Cash and cash equivalents acquired |
|
|
|
|
Net cash outflow - Investing activities |
|
|
( |
) |
Perugini S.r.l. contributed revenue of EUR
Non-controlling interests
The equity attributable to non-controlling interests at December 31, 2023 related to Medical Glass a.s. in which the Group holds an
F-30
5. Segment Information
Management identifies two operating segments, based on the internal organization and reporting structure of Stevanato Group. The criteria used to identify the Group’s operating segments are consistent with the way the chief operating decision-maker (identified in the Chief Executive Officer of Stevanato Group) assigns resources and monitors performances. They are:
The operating segments described above are also identified as reportable segments.
|
|
At and for the year ended December 31, 2023 |
|
|||||||||||||||||
|
|
Biopharmaceutical |
|
|
Engineering |
|
|
Total |
|
|
Adjustments, |
|
|
Consolidated |
|
|||||
|
|
(EUR thousand) |
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
External customers |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
||||
Inter-segment |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
— |
|
|||
Total revenue |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
Cost of sales |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
Gross Profit |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Other operating income |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
Selling and marketing expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Research and development expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
General and administrative expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Operating Profit |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
of which amortization and depreciation |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Total assets |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
Total liabilities |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
F-31
|
|
At and for the year ended December 31, 2022 |
|
|||||||||||||||||
|
|
Biopharmaceutical |
|
|
Engineering |
|
|
Total |
|
|
Adjustments, |
|
|
Consolidated |
|
|||||
|
|
(EUR thousand) |
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
External customers |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
||||
Inter-segment |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
— |
|
|||
Total revenue |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
Cost of sales |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
Gross Profit |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Other operating income |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
Selling and marketing expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Research and development expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
General and administrative expenses |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
Operating Profit |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
of which amortization and depreciation |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Total assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Total liabilities |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
|
|
At and for the year ended December 31, 2021 |
|
|||||||||||||||||
|
|
Biopharmaceutical |
|
|
Engineering |
|
|
Total |
|
|
Adjustments, |
|
|
Consolidated |
|
|||||
|
|
(EUR thousand) |
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
External customers |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
||||
Inter-segment |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
— |
|
|||
Total revenue |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
Cost of sales |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
Gross Profit |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Other operating income |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|||
Selling and marketing expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Research and development expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
General and administrative expenses |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
Operating Profit |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
of which amortization and depreciation |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
||||
Inter-segment revenue and costs are eliminated upon consolidation and reflected in the “adjustments, elimination and unallocated items” column. The most relevant adjustment in revenue relates to the sales of the Engineering’s equipment to the Biopharmaceutical and Diagnostic Solutions. “Adjustments, elimination and unallocated items” also includes some corporate residual costs not allocated to the Biopharmaceutical and Diagnostic Solutions Segment and Engineering Segment.
The reconciliation from total segments operating profit to consolidated profit before tax is as follows:
F-32
|
|
For the year ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
(EUR thousand) |
|
|||||||||
Segments Operating Profit |
|
|
|
|
|
|
|
|
|
|||
Finance income |
|
|
|
|
|
|
|
|
|
|||
Finance expense |
|
|
|
|
|
|
|
|
|
|||
Share of profit of an associate |
|
|
— |
|
|
|
— |
|
|
|
|
|
Inter-segment elimination and unallocated items |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Profit Before Tax |
|
|
|
|
|
|
|
|
|
|||
For the year ended December 31, 2023, the Group served a customer who constituted
For the years ended December 31, 2022 and 2021,
6. Revenue from contract with customers
Disaggregated revenue information
The table below shows the disaggregation of the Group’s revenue from contracts with external customers:
|
|
For the year ended December 31, 2023 |
|
|||||||||
|
|
Biopharmaceutical and Diagnostic Solutions |
|
|
Engineering |
|
|
Total |
|
|||
|
|
(EUR thousand) |
|
|||||||||
Nature of goods or service |
|
|
|
|
|
|
|
|
|
|||
Revenue from high-value solutions |
|
|
|
|
|
— |
|
|
|
|
||
Revenue from other containment and delivery solutions |
|
|
|
|
|
— |
|
|
|
|
||
Revenue from engineering |
|
|
— |
|
|
|
|
|
|
|
||
Total revenue from contracts with customers |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
Geographical markets |
|
|
|
|
|
|
|
|
|
|||
EMEA |
|
|
|
|
|
|
|
|
|
|||
APAC |
|
|
|
|
|
|
|
|
|
|||
North America |
|
|
|
|
|
|
|
|
|
|||
South America |
|
|
|
|
|
|
|
|
|
|||
Total revenue from contracts with customers |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
Timing of revenue recognition |
|
|
|
|
|
|
|
|
|
|||
Goods and services transferred at a point in time |
|
|
|
|
|
|
|
|
|
|||
Goods and services transferred over time |
|
|
|
|
|
|
|
|
|
|||
Total revenue from contracts with customers |
|
|
|
|
|
|
|
|
|
|||
F-33
|
|
For the year ended December 31, 2022 |
|
|||||||||
|
|
Biopharmaceutical and Diagnostic Solutions |
|
|
Engineering |
|
|
Total |
|
|||
|
|
(EUR thousand) |
|
|||||||||
Nature of goods or service |
|
|
|
|
|
|
|
|
|
|||
Revenue from high-value solutions |
|
|
|
|
|
— |
|
|
|
|
||
Revenue from other containment and delivery solutions |
|
|
|
|
|
— |
|
|
|
|
||
Revenue from engineering |
|
|
— |
|
|
|
|
|
|
|
||
Total revenue from contracts with customers |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
Geographical markets |
|
|
|
|
|
|
|
|
|
|||
EMEA |
|
|
|
|
|
|
|
|
|
|||
APAC |
|
|
|
|
|
|
|
|
|
|||
North America |
|
|
|
|
|
|
|
|
|
|||
South America |
|
|
|
|
|
|
|
|
|
|||
Total revenue from contracts with customers |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
Timing of revenue recognition |
|
|
|
|
|
|
|
|
|
|||
Goods and services transferred at a point in time |
|
|
|
|
|
|
|
|
|
|||
Goods and services transferred over time |
|
|
|
|
|
|
|
|
|
|||
Total revenue from contracts with customers |
|
|
|
|
|
|
|
|
|
|||
|
|
For the year ended December 31, 2021 |
|
|||||||||
|
|
Biopharmaceutical and Diagnostic Solutions |
|
|
Engineering |
|
|
Total |
|
|||
|
|
(EUR thousand) |
|
|||||||||
Nature of goods or service |
|
|
|
|
|
|
|
|
|
|||
Revenue from high-value solutions |
|
|
|
|
|
— |
|
|
|
|
||
Revenue from other containment and delivery solutions |
|
|
|
|
|
— |
|
|
|
|
||
Revenue from engineering |
|
|
— |
|
|
|
|
|
|
|
||
Total revenue from contracts with customers |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
Geographical markets |
|
|
|
|
|
|
|
|
|
|||
EMEA |
|
|
|
|
|
|
|
|
|
|||
APAC |
|
|
|
|
|
|
|
|
|
|||
North America |
|
|
|
|
|
|
|
|
|
|||
South America |
|
|
|
|
|
|
|
|
|
|||
Total revenue from contracts with customers |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
Timing of revenue recognition |
|
|
|
|
|
|
|
|
|
|||
Goods and services transferred at a point in time |
|
|
|
|
|
|
|
|
|
|||
Goods and services transferred over time |
|
|
|
|
|
|
|
|
|
|||
Total revenue from contracts with customers |
|
|
|
|
|
|
|
|
|
|||
Revenue is disclosed by nature according to the goods and services provided by our operating segments. Revenue realized by the Biopharmaceutical and Diagnostic Solutions segment includes:
The reported geographical markets are EMEA (Europe, Middle East, Africa), North America (United States, Canada, Mexico), South America and APAC (Asia Pacific). Revenue by geographical markets is based on the end customer location.
F-34
Contract balances, Trade Receivables and Advances from Customers
The following table provides information on contractual assets and liabilities from contracts with customers as well as on trade receivables and advances from customers:
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
Trade Receivables |
|
|
|
|
|
|
||
Contract Assets |
|
|
|
|
|
|
||
Contract Liabilities |
|
|
( |
) |
|
|
( |
) |
Advances From Customers |
|
|
( |
) |
|
|
( |
) |
Non-current advances from customers |
|
|
( |
) |
|
|
— |
|
The contract assets mainly relate to the Group’s right to consideration for production from construction contracts not yet invoiced as of the balance sheet date. The amounts recognized as contract assets are reclassified to trade receivable as soon as the Group has an unconditional right to consideration.
Revenue recognized in the current reporting period which relates to carried-forward contract liabilities amounts to EUR
7. Cost of sales
Cost of sales is detailed as follows:
|
|
For the year ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
(EUR thousand) |
|
|||||||||
Cost of materials |
|
|
|
|
|
|
|
|
|
|||
Direct industrial labor |
|
|
|
|
|
|
|
|
|
|||
Indirect industrial labor |
|
|
|
|
|
|
|
|
|
|||
Industrial depreciation and amortization |
|
|
|
|
|
|
|
|
|
|||
Other costs of sales |
|
|
|
|
|
|
|
|
|
|||
Total Cost of sales |
|
|
|
|
|
|
|
|
|
|||
Cost of sales consists mainly in the cost of materials, components and labor expense related to the production and distribution of our goods and services. Cost of sales also include depreciation and amortization of EUR
For the year ended December 31, 2023, cost of sales benefited from EUR
For the year ended December 31, 2022, grants received from European governments to mitigate the rise in utility cost positively affected the cost of sales by EUR
8. Other operating income
Other operating income for the year ended December 31, 2023 amounted to EUR
F-35
on the assessment performed, the Group does not consider these transactions to be part of the ordinary revenue generating activities.
9. Expenses
Expenses are detailed as follows:
|
|
For the year ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
(EUR thousand) |
|
|||||||||
Selling and marketing expenses |
|
|
|
|
|
|
|
|
|
|||
Research and development expenses |
|
|
|
|
|
|
|
|
|
|||
General and administrative expenses |
|
|
|
|
|
|
|
|
|
|||
Total Expenses |
|
|
|
|
|
|
|
|
|
|||
Selling and marketing expenses are mainly related to personnel expenses for the sales organizations and to business development and events costs, travel expenses and other marketing strategic consultancies. They also include accruals of the provision for bad and doubtful debts for EUR
Research and development expenses include costs for research and development activities to support the innovation of products and components.
General and administrative expenses consists mainly of personnel expenses for administrative functions, consultancies, directors compensation, insurance costs (such as D&O), IT expenses (such as licenses) and rental fees.
10. Other information by nature
The breakdown of the Selling and marketing, Research and development and General and administrative expenses by nature is as follows:
|
|
For the year ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
(EUR thousand) |
|
|||||||||
Personnel |
|
|
|
|
|
|
|
|
|
|||
Other costs and incomes |
|
|
|
|
|
|
|
|
|
|||
Depreciation and amortization |
|
|
|
|
|
|
|
|
|
|||
Expected credit losses |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Total expenses |
|
|
|
|
|
|
|
|
|
|||
Depreciation and amortization can be broken down as follows:
|
|
For the year ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
(EUR thousand) |
|
|||||||||
Cost of sales |
|
|
|
|
|
|
|
|
|
|||
Selling and marketing expenses |
|
|
|
|
|
|
|
|
|
|||
Research and development expenses |
|
|
|
|
|
|
|
|
|
|||
General and administrative expenses |
|
|
|
|
|
|
|
|
|
|||
Total depreciation & amortization |
|
|
|
|
|
|
|
|
|
|||
F-36
Depreciation and amortization recognized among general and administrative expenses include amortization of fair value adjustments from purchase price allocations on trademarks related to Balda Group companies amounting to EUR
For further details on amortization and depreciation for the years ended December 31, 2023 and 2022, reference should be made to the movements in property, plant and equipment, intangible assets and right of use assets (Note 17 - 18 - 34).
11. Finance income
Finance income are as follows:
|
|
For the years ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
(EUR thousand) |
|
|||||||||
Interest income from banks deposits |
|
|
|
|
|
|
|
|
|
|||
Income from financial discounts |
|
|
|
|
|
|
|
|
|
|||
Interest income on loans to associates |
|
|
— |
|
|
|
— |
|
|
|
|
|
Other financial income |
|
|
|
|
|
|
|
|
|
|||
Gain from the sale of an associate |
|
|
— |
|
|
|
— |
|
|
|
|
|
Foreign currency exchange rate gains |
|
|
|
|
|
|
|
|
|
|||
Derivatives revaluation |
|
|
|
|
|
|
|
|
|
|||
Other fair value adjustments |
|
|
|
|
|
|
|
|
|
|||
Total finance income |
|
|
|
|
|
|
|
|
|
|||
For the year ended December 31, 2021 the Group realized a gain of EUR
12. Finance expense
Finance expense are as follows:
|
|
For the year ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
(EUR thousand) |
|
|||||||||
Interest on debts and borrowings |
|
|
|
|
|
|
|
|
|
|||
Financial discounts and other expenses |
|
|
|
|
|
|
|
|
|
|||
Interest on lease liabilities |
|
|
|
|
|
|
|
|
|
|||
Financial component IAS 19 |
|
|
|
|
|
|
|
|
|
|||
Foreign currency exchange losses |
|
|
|
|
|
|
|
|
|
|||
Derivatives devaluation |
|
|
|
|
|
|
|
|
|
|||
Other fair value adjustments |
|
|
|
|
|
|
|
|
|
|||
Total finance expense |
|
|
|
|
|
|
|
|
|
|||
Finance expenses include bank interest on the Group’s financial debt (calculated using the effective interest method) and interest on leases about the portion of financial expenses payable matured in the reporting period on the liabilities, recognized in accordance with IFRS 16 - Leases.
Foreign exchange differences are realized and unrealized gains and losses incurred on transactions in currencies other than the functional currency of the Group; the net foreign currency exchange impact, given by the sum of gains and losses, amounted to EUR
Derivatives revaluation and derivatives devaluation include changes in the fair values of the foreign currency forward contracts that have not been designated as hedge accounting relationships as well as the ineffectiveness of the foreign currency forward contracts designated in a cash flow hedge.
F-37
13. Employee benefits expense
Employee benefits expense are detailed as follows:
|
|
For the year ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
(EUR thousand) |
|
|||||||||
Included in Cost of sales: |
|
|
|
|
|
|
|
|
|
|||
Wages and salaries |
|
|
|
|
|
|
|
|
|
|||
Social security costs |
|
|
|
|
|
|
|
|
|
|||
Pension costs |
|
|
|
|
|
|
|
|
|
|||
Share-based payment expense |
|
|
|
|
|
|
|
|
— |
|
||
Included in Selling and Marketing expenses: |
|
|
|
|
|
|
|
|
|
|||
Wages and salaries |
|
|
|
|
|
|
|
|
|
|||
Social security costs |
|
|
|
|
|
|
|
|
|
|||
Pension costs |
|
|
|
|
|
|
|
|
|
|||
Share-based payment expense |
|
|
|
|
|
|
|
|
— |
|
||
Included in Research and Development expenses: |
|
|
|
|
|
|
|
|
|
|||
Wages and salaries |
|
|
|
|
|
|
|
|
|
|||
Social security costs |
|
|
|
|
|
|
|
|
|
|||
Pension costs |
|
|
|
|
|
|
|
|
|
|||
Share-based payment expense |
|
|
|
|
|
|
|
|
— |
|
||
Included in General and Administrative expenses: |
|
|
|
|
|
|
|
|
|
|||
Wages and salaries |
|
|
|
|
|
|
|
|
|
|||
Social security costs |
|
|
|
|
|
|
|
|
|
|||
Pension costs |
|
|
|
|
|
|
|
|
|
|||
Cash settled awards |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Share-based payment expense |
|
|
|
|
|
|
|
|
|
|||
Total employee benefits expense |
|
|
|
|
|
|
|
|
|
|||
The average size of the Group's workforce during the year is as follows:
|
|
For the year ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Executives |
|
|
|
|
|
|
|
|
|
|||
Managers |
|
|
|
|
|
|
|
|
|
|||
Employees |
|
|
|
|
|
|
|
|
|
|||
Total Workforce |
|
|
|
|
|
|
|
|
|
|||
14. Income tax
Income tax expense is as follows:
|
|
For the year ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
(EUR thousand) |
|
|||||||||
Current income tax: |
|
|
|
|
|
|
|
|
|
|||
Current Taxes |
|
|
|
|
|
|
|
|
|
|||
Prior Years Taxes |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
Deferred tax: |
|
|
|
|
|
|
|
|
|
|||
Deferred Taxes |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
Income tax expense reported in the statement of profit or loss |
|
|
|
|
|
|
|
|
|
|||
F-38
|
|
For the year ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
(EUR thousand) |
|
|||||||||
Deferred tax related to items recognized in OCI during in the year: |
|
|
|
|
|
|
|
|
|
|||
Gains/(losses) from remeasurement of employee of defined benefit plans and of agent termination plans |
|
|
|
|
|
( |
) |
|
|
|
||
Change in the fair value of hedging instruments |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Deferred tax charged to OCI |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
The table below provides a reconciliation between actual income tax expense and the theoretical income tax expense, calculated on the basis of the applicable corporate tax rate in effect in Italy.
|
|
For the year ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
(EUR thousand) |
|
|||||||||
Accounting profit before income tax |
|
|
|
|
|
|
|
|
|
|||
Statutory income tax rate of |
|
|
|
|
|
|
|
|
|
|||
Prior years taxes |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
DTA not recognized on tax losses carry-forward |
|
|
|
|
|
|
|
|
( |
) |
||
DTA on temporary/tax losses non recognized |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
Taxes effect on unremitted earnings |
|
|
|
|
|
|
|
|
|
|||
Tax grants/not taxable items |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Tax exemption on gain from the sale of an associate |
|
— |
|
|
|
— |
|
|
|
( |
) |
|
Different foreign tax rate effect |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
DTA/DTL effect previous years |
|
|
|
|
|
— |
|
|
|
— |
|
|
At the effective income tax rate of |
|
|
|
|
|
|
|
|
|
|||
Income tax expense reported in the statement of profit or loss |
|
|
|
|
|
|
|
|
|
|||
The Group's effective tax rate for the year ended December 31, 2023, decreased to
Unrecognized tax losses at December 31, 2023 and at December 31, 2022 amounted to EUR
F-39
The breakdown on the timing of tax losses carry-forwards is as follows:
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
Timing of unrecognized tax losses carry-forwards |
|
|
|
|
|
|
||
2023 |
|
— |
|
|
|
|
||
2024 |
|
— |
|
|
|
|
||
2025 |
|
— |
|
|
|
|
||
2026 |
|
— |
|
|
|
|
||
2027 |
|
— |
|
|
|
|
||
2028 |
|
— |
|
|
|
|
||
2029 |
|
— |
|
|
|
|
||
Unlimited |
|
|
|
|
|
|
||
Total unrecognized tax losses |
|
|
|
|
|
|
||
The change in unrecognized tax losses is related to both the recognition of deferred tax assets and the use of tax losses expiring within 2029.
The analysis of deferred tax assets and deferred tax liabilities as at December 31, 2023 and 2022 is as follows:
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
Intangible assets |
|
|
( |
) |
|
|
( |
) |
Property, plant and equipment |
|
|
|
|
|
|
||
Contract balances |
|
|
( |
) |
|
|
( |
) |
Revaluations of investment properties to fair value |
|
|
|
|
|
|
||
Expected credit losses |
|
|
|
|
|
|
||
Derivatives |
|
|
( |
) |
|
|
( |
) |
Leases |
|
|
|
|
|
|
||
Long term incentives |
|
|
|
|
|
|
||
Provisions |
|
|
|
|
|
|
||
Accruals and other provisions |
|
|
|
|
|
|
||
Tax losses carry forward |
|
|
|
|
|
|
||
Unremitted earnings |
|
|
( |
) |
|
|
( |
) |
Start up costs IPO SG spa |
|
|
|
|
|
|
||
Share-based compensation plans |
|
— |
|
|
|
|
||
Other effects |
|
|
|
|
|
|
||
Deferred tax assets, net |
|
|
|
|
|
|
||
Reflected in the statement of financial position as follows: |
|
|
|
|
|
|
||
Deferred tax assets |
|
|
|
|
|
|
||
Deferred tax liabilities |
|
|
( |
) |
|
|
( |
) |
Deferred tax assets, net |
|
|
|
|
|
|
||
Deferred taxes are calculated based on the global allocation criteria, taking into account the cumulative amount of all the temporary differences, based on the average expected rates in force when these temporary differences reverse.
With reference to Deferred Tax Assets (DTA) on net operating tax loss (“NOL”) carryforwards, at December 31, 2023 the Group recognized DTA on NOL of EUR
The Group believes that it is probable that sufficient future taxable profits will be generated to support the recognized deferred tax asset for tax losses carried forward in all jurisdictions. As part of its recoverability assessment the Group has taken into account (i) the most recent forecast approved by management and the Board of Directors, (ii) the likelihood that the factors that have contributed to past losses in some countries will not recur, (iii), the future reversal of existing taxable
F-40
temporary differences, (iv) the legal right to carryforward and utilization without time limit (some restrictions on yearly use may occur).
The Group has applied the temporary exception issued by the IASB in May 2023 from the accounting requirements for deferred taxes in IAS 12.
Accordingly, the Group neither recognizes nor discloses information about deferred tax assets and liabilities related to Pillar Two income taxes. On December 28, 2023, the government of Italy, where the parent company is incorporated for tax purposes, enacted the Pillar Two income taxes legislation effective from January 1, 2024 (see Legislative Decree no. 209/2023, hereinafter “the Italian Pillar Two rules”). According to the Italian Pillar Two rules, Stevanato Holding S.r.l. qualifies as the ultimate parent entity (“UPE”) for Pillar Two purposes, as it consolidates Stevanato Group S.p.A. on a line-by-line basis. As a consequence, the Pillar Two perimeter would be identified with that of the consolidated financial statements of Stevanato Holding S.r.l., including all the entities which are consolidated on a line-by-line basis. As the UPE, Stevanato Holding S.r.l. will be in charge of the calculation of the jurisdictional effective tax rate according to the Pillar Two Rules. Stevanato Holding S.r.l. directly holds only the controlling participation in Stevanato Group S.p.A. at
Under the Italian Pillar Two rules, the UPE will be generally required to pay, in Italy, a top-up tax on profits of its subsidiaries that are taxed at an effective tax rate (determined in accordance with the Italian Pillar Two rules) of less than 15%. The Group has performed a preliminary assessment of the “Transitional Safe Harbours” for Pillar Two purposes ("TSH") on the basis of the OECD rules on “Safe Harbour and Penalty Relief” issued on December 20, 2022, which are intended as “qualifying international agreement on safe harbours” for the purposes of the EU Directive n. 2523/2022 (art. 32) and the Italian Pillar Two rules. This preliminary assessment is based on the 2022 Country by Country Reporting ("CbCR") of the Group and accounting data for fiscal year 2022. Based on fiscal year 2022 financial data, the main jurisdictions in which a potential exposure to top-up-tax may exist include China and Mexico, as no TSH test would be met. However, as the effective tax rate calculated for TSH purposes is close to 15%, no significant impact in terms of potential top up tax is expected. For the sake of completeness, we highlight that currently China and Mexico have not implemented yet a local Qualified Domestic Top up Tax within their domestic legislation. This preliminary assessment has been performed considering a number of technical positions based on the content of the TSH rules and other guidelines currently available. In this regard, considering the lack of specific interpretations and explanations by the OECD, the EU Directive, the Italian law, such technical positions shall be confirmed once the expected clarifications will be provided at OECD, EU and domestic level. Because this preliminary assessment serves only to provide general guidance, the actual impact that the Pillar Two legislation would have had on the Group’s results if it had been in effect for the year ending December 31, 2023 may have been significantly different. The Group is continuing to assess the impact of the Pillar Two income tax legislation on its future financial performance.
The reconciliation of net deferred tax assets is as follows:
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
As of January 1 |
|
|
|
|
|
|
||
Tax expense during the period recognized in profit or loss |
|
|
|
|
|
|
||
Tax income/(expense) during the period recognized in OCI |
|
|
|
|
|
( |
) |
|
Other effect |
|
|
|
|
|
|
||
As at December 31 |
|
|
|
|
|
|
||
The other effect movement includes foreign exchange differences and minor reclassification.
15. Earnings per Share
Basic earnings per share (EPS) is calculated by dividing the profit attributable to equity holders of the parent by the weighted average number of shares issued net of the treasury shares held by the Group.
The weighted average number of shares for diluted earnings per share was increased to take into consideration the theoretical effect of potential ordinary shares that would be assigned to the beneficiaries based on the Group’s share-based incentive plans (see Notes 28 and 29 for further details on the share-based incentive plans).
F-41
The following table reflects the income and share data used in the basic and diluted EPS calculation:
|
|
At December 31, |
|
|
At December 31, |
|
|
At December 31, |
|
|||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
|
|
|
|
|
|
|
|
|||
Profit attributable to ordinary equity holders of the parent (in EUR thousand) |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
Weighted average number of ordinary shares for basic EPS |
|
|
|
|
|
|
|
|
|
|||
Effects of dilution from share-based incentive plans |
|
|
|
|
|
— |
|
|
|
|
||
Effects of dilution from remuneration in shares |
|
|
|
|
|
|
|
|
— |
|
||
Weighted average number of ordinary shares adjusted for the effect of dilution |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Basic earnings per common share (in EUR) |
|
|
|
|
|
|
|
|
|
|||
Diluted earnings per common share (in EUR) |
|
|
|
|
|
|
|
|
|
|||
16. Goodwill
In accordance with IAS 36 - Impairment of assets, Goodwill is tested for impairment annually, or more frequently if facts or circumstances indicate that the asset may be impaired. Impairment testing is performed by comparing the carrying amount and the recoverable amount of the CGU (or groups of CGUs) to which it is allocated. The recoverable amount of the CGU is the higher of its fair value less costs of disposal and its value in use.
Until 2022, for the purpose of the impairment test on goodwill, management identified
For 2023, impairment test of goodwill is performed at the level corresponding to the operating segments (refer to Note 5), which is the lowest level at which goodwill is monitored and performances assessed.
The change in impairment testing approach is due to the implementation of a new internal organization and the way management monitors goodwill, consequence of the increasingly cross-linked activities between companies and resources belonging to the IVD&DDS and DCS businesses and the evolution of the business and increase of activities for the DDS which have spread across the BDS segment.
For the purpose of impairment testing, goodwill is allocated as follows:
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
Biopharmaceutical and Diagnostic Solutions (*) |
|
|
|
|
|
|
||
Engineering Systems |
|
|
|
|
|
|
||
Total Goodwill |
|
|
|
|
|
|
||
(*)
The change in the item "Engineering Systems" of EUR
F-42
The objective of the impairment test is to compare the recoverable amount of each CGU with their corresponding carrying amount of net assets including goodwill. The recoverable amount is the higher of an asset’s fair value less costs to sell and its value in use. The Group determines the value in use of the CGU to which the goodwill refers, meaning the present value of the future cash flows expected to be derived from continuous use of the assets; any cash flows arising from extraordinary events are therefore ignored.
The impairment test is performed every year at year end.
The value in use has been determined by applying the Discounted Cash Flow ("DCF") method. This method has been applied with a two-stage approach, the first corresponding to the explicit forecast period (2024-2029) and the second corresponding to a terminal value derived with inertial criteria for the period after 2029. The explicit period corresponds with the horizon covered by the budget and the plans prepared by management and approved by the Audit Committee, assuming realistic scenarios on the information available at the reporting date, including macroeconomic indicators and geo-political trends.
The principal assumptions adopted by management in drawing up the projections relates mainly to a growth in volumes of products and different product mixes, shifting to high-value solutions sales, expanding the SG EZ-fill® industrial footprint to address customer proximity and reshoring needs, completing the development of the DDS proprietary product portfolio and development CDMO opportunities, and continuing business optimization efforts in engineering. Volumes and sales mix used for estimating the future cash flows are based on assumptions that are considered reasonable and sustainable and represent the best estimate of expected conditions regarding market trends for the CGUs over the period considered.
The growth rate in terminal value used for projecting beyond the explicit planning period (2024-2029) is
The cash flows and discount rate were determined net of tax. Future cash flows are discounted using the weighted average cost of capital (WACC); this is estimated with a beta factor derived on the basis of a peer group. The discount rates,
The results obtained using the discounted cash flow method have been tested for their sensitivity to changes in certain key variables, within reasonable ranges. The variables altered were (i) the WACC (between
Finally, the discount rate and variation to the forecast EBITDA at continuing value within the impairment test have been identified that would allow a value in use equal to the carrying amount of the net assets of the respective CGU. This further sensitivity analysis resulted in the identification of breakeven for the BDS CGU with a WACC of
The impairment test for the goodwill did not result in any need for impairment.
Even though the above described reorganization, that determined the reallocation of goodwill, does not consist in a restructuring and does not represent an impairment trigger, the impairment test has been also performed under the old structure of CGUs to which goodwill was allocated, without identifying any impairment loss.
WACC has been calculated equal to
F-43
17. Intangible assets
Changes in intangible assets for the year ended December 31, 2023 are as follows:
|
|
Development |
|
|
Industrial |
|
|
Concessions, |
|
|
Intangible |
|
|
Other |
|
|
Total |
|
||||||
|
|
(EUR thousand) |
|
|||||||||||||||||||||
Cost |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
At January 1, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Additions |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Disposals |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Reclassifications |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|||||
Exchange differences |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||||
At December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Additions |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Disposals |
|
|
|
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
|
|
|
( |
) |
|||
Reclassifications |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|||||
Exchange differences |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
At December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Amortization |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
At January 1, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Amortization |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Disposal |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Exchange differences |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||||
At December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Amortization |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Disposal |
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
||||
Exchange differences |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
( |
) |
||
At December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Net book value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
At December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
At December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Development costs are referred to costs for the study, design and prototype development for products which have been or are expected to be commercialized and for which is probable that the expected future economic benefits will flow to the entity. Development expenses are recognized in the consolidated income statement as research and development expenses.
Industrial patents and intellectual property rights increase for EUR
Concessions, licenses, trademarks and similar rights with a total carrying amount of EUR
Intangible fixed assets in process and advances refer to ongoing projects which will conclude in subsequent years. Intangible fixed assets and advances increase for EUR
No impairment indicators have been identified for intangible assets and therefore
F-44
18. Property, plant and equipment
Changes in items of property, plant and equipment in 2023 are as follows:
|
|
Land and |
|
|
Plant and |
|
|
Industrial |
|
|
Other |
|
|
Assets under |
|
|
Total |
|
||||||
|
|
(EUR thousand) |
|
|||||||||||||||||||||
Cost |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
At January 1, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Additions |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Disposals |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Reclassifications |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
— |
|
||||
Exchange differences |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|||||
At December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Additions |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Acquisition of Perugini S.r.l. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|||||
Disposals |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
Reclassifications |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|||||
Exchange differences |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
||||
At December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Depreciation and impairment |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
At January 1, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|||||
Depreciation charge for the year |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|||||
Impairment |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Disposals |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
Exchange differences |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|||||
At December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|||||
Depreciation charge for the year |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|||||
Acquisition of Perugini S.r.l. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|||||
Reclassifications |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
Disposals |
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
Exchange differences |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|||||
At December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Net book value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
At December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
At December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Increase in Land and buildings at December 31, 2023 was mainly related to the grant of land by the city of Fishers (Indiana, U.S.), amounting to EUR
At December 31, 2023 the overall increases in plant and machinery, considering both the yearly additions and the reclassification from assets under construction, amounted to EUR
Assets under construction, included investments in production lines and machines for syringes, vials and cartridges production which have not yet been completed but are expected to enter into use in the coming years. This category also
F-45
included: (i) the investment for the new EZ-fill® plant in Latina, (ii) the investment for the new EZ-fill® hub in China, (iii) the investments for completing the new building in Piombino Dese, that will host corporate offices, production lines and the EMEA TEC Center and (iv) the investments for the construction of the new U.S. facility in Fishers, Indiana.
At December 31, 2023 committed orders related to the ongoing investments equaled approximately EUR
At December 31, 2023, EUR
At December 31, 2023, approximately EUR
At the year end,
19. Financial assets
The following table details the composition of financial assets:
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
Fair value of derivatives financial instruments |
|
|
|
|
|
|
||
Other non-current financial assets |
|
|
|
|
|
|
||
Other non-current financial assets |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Fair value of derivatives financial instruments |
|
|
|
|
|
|
||
Life insurance policies |
|
|
— |
|
|
|
|
|
Other current financial assets |
|
|
|
|
|
— |
|
|
Other current financial assets |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Other Financial Assets |
|
|
|
|
|
|
||
At December 31, 2022, other current financial assets included life insurance policies managed by Société Générale SA, which were measured at fair value. The decrease in other current financial assets of EUR
At December 31, 2023, other non-current financial assets included a secured senior convertible promissory note amounting to EUR
At December 31, 2023 and 2022, other non-current financial assets and other current financial assets included interest rate swap derivatives. Other current financial assets also include foreign exchange derivatives. At December 31, 2023 interest
F-46
rate swap derivatives were included in other non-current financial liabilities while commodity swap derivatives were included in other current financial liabilities.
The following table sets further the analysis of derivative assets and liabilities at December 31, 2023 and December 31, 2022.
|
|
At December 31, |
|
|
At December 31, |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
||||||||||
|
|
Carrying |
|
|
Fair |
|
|
Carrying |
|
|
Fair |
|
||||
|
|
(EUR thousand) |
|
|||||||||||||
Non-Current financial assets |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Interest Rate Swap - hedging instruments |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Current financial assets |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Foreign exchange forward contracts - trading derivatives |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Foreign exchange forward contracts - hedging instruments |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Interest Rate Swap - hedging instruments |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Non-Current financial liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Interest Rate Swap - hedging instruments |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Current financial liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Commodity Swap - hedging instruments |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
At December 31, 2023 and 2022 part of the derivatives on currency risk have not been designated as hedging instruments and reflect the change in the fair value of those foreign exchange forward contracts that are not designated in hedge relationships, but are, nevertheless, intended to reduce the level of foreign currency risk for expected sales. The change in the fair value of the derivatives not designed as hedging instruments is recorded among finance income and finance expense.
Derivatives designated as hedging instruments reflect the change in fair value of
- the interest rate swap contracts, designated as cash flow hedges to hedge fluctuations in variable interest rate on loans;
- the foreign exchange forward contracts, designed as cash flow hedges to hedge highly probable forecast sales in U.S. Dollars;
- the commodity swap contracts, designed to hedge against price swings in the market for natural gas and electricity.
The change in the fair value of the derivatives designed as hedging instruments is recorded in a separate component of equity (cash flow hedge reserve). The amount recorded in the cash flow hedge reserve will be recognized in the consolidated income statement according to the timing of the cash flows of the underlying transaction.
At the year end,
20. Inventories
Inventories, shown net of an allowance for obsolete and slow-moving goods, can be analyzed as follows:
F-47
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
Raw materials |
|
|
|
|
|
|
||
Semifinished products |
|
|
|
|
|
|
||
Finished products |
|
|
|
|
|
|
||
Provision from slow moving and obsolescence |
|
|
( |
) |
|
|
( |
) |
Total inventories |
|
|
|
|
|
|
||
Raw material includes advance paid to suppliers for inventories in the amount of EUR
Changes in the provision for slow moving and obsolete inventories are as follows:
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
At January 1 |
|
|
|
|
|
|
||
Provision |
|
|
|
|
|
|
||
Utilizations and other changes |
|
|
( |
) |
|
|
( |
) |
At December 31 |
|
|
|
|
|
|
||
21. Trade receivables and contract assets
Trade receivables and contract assets are analyzed as follows:
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
Trade receivables |
|
|
|
|
|
|
||
Allowance for expected credit losses |
|
|
( |
) |
|
|
( |
) |
Total trade receivables |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Trade receivables are non-interest bearing and credit terms are generally
Trade receivables breakdown by geographical area is shown below:
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
EMEA |
|
|
|
|
|
|
||
APAC |
|
|
|
|
|
|
||
North America |
|
|
|
|
|
|
||
South America |
|
|
|
|
|
|
||
Total Trade Receivables |
|
|
|
|
|
|
||
F-48
Trade receivables are stated net of an allowance for expected credit losses which has been determined in accordance with IFRS 9 amounting to EUR
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
At January 1 |
|
|
|
|
|
|
||
Accruals |
|
|
|
|
|
|
||
Releases |
|
|
( |
) |
|
|
( |
) |
Utilizations |
|
|
( |
) |
|
|
( |
) |
Exchange differences and other changes |
|
|
|
|
|
|
||
At December 31 |
|
|
|
|
|
|
||
Contract assets
Contract assets relate to ongoing customer-specific construction contracts within the Engineering segment and from the In-vitro diagnostic business, which is part of the Biopharmaceutical and Diagnostic Solutions Segment. As such, the balances of this account vary and depend on the number of ongoing construction contracts at the end of the year. The Group had contract assets of EUR
22. Tax receivables and tax payables
The breakdown in the account is as follows:
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
Tax receivables |
|
|
|
|
|
|
||
Tax payables |
|
|
( |
) |
|
|
( |
) |
Tax receivables decreased mainly due to (i) the use of energy tax credit accrued by Italian legal entities for fiscal year 2022 and (ii) the payment by Stevanato Holding S.r.l. (the parent company in the group taxation regime) of the full amount of the Stevanato Group’s tax losses, transferred within the consolidated tax group. Participation in the Italian tax consolidation regime enables each participating company to optimize the financial management of corporate income tax, for example by netting the taxable income and tax losses of each participant within the tax group.
Tax receivables at December, 31 2023 were mainly composed by the tax credit of Stevanato Group S.p.A. for the tax loss for fiscal year 2023 (EUR
Tax payables decreased mainly due to the payments made by Italian legal entities to Stevanato Holding S.r.l. as settlement of fiscal year 2022 income tax payable and advance payment for fiscal year 2023.
F-49
23. Other receivables
Other receivables are as follows:
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
Advances to suppliers |
|
|
|
|
|
|
||
Accrued income and prepayments |
|
|
|
|
|
|
||
VAT receivables |
|
|
|
|
|
|
||
Other receivables |
|
|
|
|
|
|
||
Total other receivables |
|
|
|
|
|
|
||
24. Equity
The main objective of the Group’s capital management is to guarantee maintenance of a solid credit rating and adequate financial ratios with a view to supporting business activity and maximizing value for the shareholders.
Capital Management
The Group’s objectives when managing capital are to create value for shareholders as a whole, safeguard business continuity and support the sustainable growth of the Group. As a result, the Group endeavors to maintain a satisfactory economic return for its shareholders and guarantee economic access to external sources of funds.
Share capital
At December 31, 2023 and 2022 the Company paid-in share capital amounted to EUR
The dual class structure of our shares includes ordinary shares and Class A shares. The Class A shares have the same characteristics and grant the shareholders the same rights as the ordinary shares, except for the voting rights. Holders of ordinary shares are entitled to one vote per share, while holders of Class A shares (held solely by Stevanato Holding S.r.l. or held in treasury by the Company) are entitled to three votes per share. The Class A shares are automatically converted (without the need for a resolution by the special meeting of the shareholders holding Class A shares or by the shareholders’ meeting) into ordinary shares, at a ratio of one ordinary share for each Class A share, in the event of transfer to parties other Stevanato Family members, or other than companies or other entities controlled, including jointly, directly or indirectly, by one or more members of the Stevanato Family, or by trusts (or, alternatively, by the relevant trustees) set up by members of the Stevanato Family, provided that the relevant beneficiaries are (or may be) one or more members of the Stevanato Family. The Class A shares are convertible into ordinary shares, at a ratio of one ordinary share for each Class A share, in whole or in part and even in several tranches, at the simple request of each holder, to be submitted by means of a communication sent by registered letter, e-mail or any other means capable of providing proof of receipt to the chairman of the board of directors of the Company, with a copy to the chairman of the Audit Committee. Under no circumstances may ordinary shares be converted into Class A shares.
Share Premium Reserve
The share premium reserve includes the additional paid-in capital raised during the Initial Public Offering net of the listing costs pertaining to the public subscription offer to the extent they were incremental costs directly attributable to the equity transaction that otherwise would have been avoided. At December 31, 2023 and 2022 the share premium reserve amounted to EUR
Treasury shares
At December 31, 2022 a total of
F-50
shares awarded to the beneficiaries of share based incentive plans, a total of
Cash flow hedge reserve
The cash flow hedge reserve reflects the negative change in fair value of derivatives financial instruments, designated as cash flow hedges. At December 31, 2023 the cash flow hedge reserve amounted to EUR
Cost of hedging reserve
The cost of hedging reserve reflects the forward element of forward contracts. At December 31, 2023 the cost of hedging reserve amounted to EUR (
Reserve for actuarial gains/losses
The reserve for actuarial gains/losses includes actuarial gains and losses on the net defined employees benefits liability and on the agents termination plans. At December 31, 2023 the reserve for actuarial gains/losses amounted to EUR (
Currency translation reserve
The currency translation reserve includes the cumulative foreign currency translation differences arising from the translation of financial statements denominated in currencies other than Euro; at December 31, 2023 it amounted to EUR (
Retained earnings and other reserves
Retained earnings and other reserves include:
Net profit attributable to equity holders of the parent
Net Profit attributable to equity holders of the parent amount to EUR
Non-controlling interests
Non-controlling interests amount to EUR
25. Dividends
On May 24, 2023 Stevanato Group shareholders approved the distribution of EUR
F-51
and the remaining balance, which related to dividends payables to Stevanato Holding S.r.l., was paid in the fourth quarter of 2023.
On June 1, 2022 Stevanato Group shareholders approved the distribution of EUR
26. Financial liabilities
Total financial liabilities are EUR
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
Lease liabilities |
|
|
|
|
|
|
||
Bank overdrafts and short-term loan facilities |
|
|
|
|
|
|
||
Bank loans |
|
|
|
|
|
|
||
Financial liabilities due to related parties |
|
|
— |
|
|
|
|
|
Fair value of derivatives |
|
|
|
|
|
— |
|
|
Financial payables for shares acquisition |
|
|
|
|
|
— |
|
|
Financial liabilities due to other lenders |
|
|
— |
|
|
|
|
|
Financial liabilities for accrued interests |
|
|
|
|
|
— |
|
|
Total current financial liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Lease liabilities |
|
|
|
|
|
|
||
Bank loans |
|
|
|
|
|
|
||
Notes |
|
|
|
|
|
|
||
Fair value of derivatives |
|
|
|
|
|
— |
|
|
Total non-current financial liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Financial Liabilities |
|
|
|
|
|
|
||
On April 16, 2020 Stevanato Group entered into a note purchase and private shelf agreement with PGIM, Inc. and certain of its affiliates, pursuant to which, for a period of
The Note Purchase Agreement imposes certain covenants on the Group, including: (i) the notes must always rank at least pari passu with all other unsecured and unsubordinated indebtedness of the company and the guarantor; (ii) any covenant included in a different financing agreement which is more favorable to the lenders must apply to the Note Purchase Agreement, as well; (iii) the aggregate EBITDA of the company and the guarantor must always be at least equal to a certain percentage of the EBITDA of our group; (iv) no merger or consolidation for any guarantor unless expressly permitted by the Note Purchase Agreement; (v) no dealings with sanctioned entities; (vi) the ratio of consolidated net debt to consolidated EBITDA not to be greater than
As at December 31, 2023 and 2022, all financial covenants are complied with.
At December 31, 2023, the bank loans amounted to a total of EUR
F-52
floating rates and have a
The increase in bank overdrafts and short term loans was mainly due to the taking out of some short-term financing.
The following table sets forth the reconciliation of total borrowings:
|
|
At December 31, |
|
|
Cash flows |
|
|
Non-cash changes |
|
|
At December 31, |
|
||||||||||||||||
|
|
2022 |
|
|
Proceeds |
|
|
Repayments |
|
|
Exchange rate |
|
|
Amortized Cost |
|
|
Accrued Interests |
|
|
2023 |
|
|||||||
|
|
(EUR thousand) |
|
|||||||||||||||||||||||||
Bank loans |
|
|
|
|
|
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
|
|
|
|
|
||||
Bank overdrafts and short-term loan facilities |
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
|
|
|
|
||||
Notes |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|||
Financial liabilities due to related parties |
|
|
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Financial liabilities due to other lenders |
|
|
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total Borrowings |
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||||
The following table shows maturities and average interest rates for liabilities to banks and other lenders:
At December 31, 2023
|
|
Currency |
|
Amount |
|
Maturity |
|
Average |
|
Amount in EUR |
Bank Loans |
|
|
|
|
|
|||||
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|||||
Amortized Cost |
|
|
( |
|
|
|
|
( |
||
Total Bank Loans |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes |
|
|
|
|
|
|||||
|
|
|
|
|
|
|||||
Amortized Cost |
|
|
( |
|
|
|
|
( |
||
Total Notes |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Overdrafts and short-term loan facilities |
|
|
|
|
|
|||||
|
|
|
|
|
|
|||||
Total Overdrafts and short-term loan facilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Bank Loans and Overdrafts |
|
|
|
|
|
|
|
|
|
F-53
At December 31, 2022
|
|
Currency |
|
Amount |
|
Maturity |
|
Average |
|
Amount in EUR |
Bank Loans |
|
|
|
|
|
|||||
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|||||
Amortized Cost |
|
|
( |
|
|
|
|
( |
||
Total Bank Loans |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes |
|
|
|
|
|
|||||
|
|
|
|
|
|
|||||
Amortized Cost |
|
|
( |
|
|
|
|
( |
||
Total Notes |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Overdrafts and short-term loan facilities |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
Total Bank Loans and Overdrafts |
|
|
|
|
|
|
|
|
|
Financial liabilities require compliance with certain covenants on the Group consolidated figures, including: (i) not to exceed certain consolidated net debt to consolidated EBITDA ratios (not greater than
At December 31, 2023 and 2022, all financial covenants are complied with.
Some short-term payables are subject to secured guarantee. Please refer to Note 37.
27. Fair Value Measurement
The following table shows the fair value hierarchy for financial assets and liabilities that are measured at fair value on a recurring basis at December 31, 2023:
|
|
|
|
Fair value measurement using |
|
|||||||||||||
|
|
Notes |
|
Total |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
||||
|
|
|
|
(EUR thousand) |
|
|||||||||||||
Cash and cash equivalents |
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
||
Financial assets - investments FVTPL - traded |
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
||
Financial assets - investments FVTPL - not traded |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Non-current financial assets - derivatives |
|
19 |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
||
Current financial assets - derivatives |
|
19 |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
||
Other current financial assets |
|
19 |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
||
Other non-current secured notes at FVTPL |
|
19 |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Total assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Current financial liabilities - derivatives |
|
26 |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
||
Non-current financial liabilities - derivatives |
|
26 |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
||
Total Liabilities |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
||
F-54
At December 31, 2022:
|
|
|
|
Fair value measurement using |
|
|||||||||||||
|
|
Notes |
|
Total |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
||||
|
|
|
|
(EUR thousand) |
|
|||||||||||||
Cash and cash equivalents |
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
||
Financial assets - investments FVTPL - traded |
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
||
Financial assets - investments FVTPL - not traded |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Current financial assets - derivatives |
|
19 |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
||
Non-current financial assets - derivatives |
|
19 |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
||
Life insurance policies |
|
19 |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
||
Other non-current financial assets |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
||
Total assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
The fair value of current financial assets and other financial liabilities is measured by taking into consideration market parameters at the balance sheet date and using valuation techniques widely accepted in the financial business environment.
The fair value of foreign currency derivatives (forward contracts, currency swaps and options) is determined by considering the present value of future cash flows based on the forward exchange rates at the reporting date. The fair value of interest rate swaps is determined by considering the present value of the estimated future cash flows based on observable yield curves. The fair value of commodity swaps is tied to the market price of the underlying commodities Italian electricity "Prezzo Unico Nazionale" (PUN) and natural gas "Punto di Scambio Virtuale" (PSV-Day Ahead Price - Heren) at the balance sheet date.
Although cash and cash equivalents are measured at amortized cost, the value approximates fair value due to the short maturity of these instruments, which consist of bank current accounts. The fair value of other non-current financial assets is measured through other unobservable input in accordance with IFRS 13.
The following table presents the changes in level 3 instruments for the year ended December 31, 2023:
|
|
Financial assets - investments FVTPL - not traded |
|
|
Non-current secured notes at FVTPL |
|
|
Total |
|
|||
|
|
(EUR thousand) |
|
|||||||||
At December 31, 2022 |
|
|
|
|
|
— |
|
|
|
|
||
Additions |
|
|
|
|
|
|
|
|
|
|||
At December 31, 2023 |
|
|
|
|
|
|
|
|
|
|||
The increase in non-current secured notes at FVTPL is related to a secured senior convertible promissory note amounting to EUR
There were
The fair value of the loans accounted for at amortized cost approximates their carrying amounts as of December 31, 2023 and 2022.
F-55
28. Employee benefits
Employee benefits are analyzed as follows:
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
Employee severance indemnity |
|
|
|
|
|
|
||
Jubilee benefits |
|
|
|
|
|
|
||
Other post-employment plans |
|
|
|
|
|
|
||
Long term incentive plan |
|
|
— |
|
|
|
|
|
Stock grant plan |
|
|
|
|
|
|
||
Other share-based compensation |
|
|
— |
|
|
|
|
|
Total employee benefits |
|
|
|
|
|
|
||
Defined benefit obligations - Italian employee severance indemnity (TFR)
Trattamento di fine rapporto or “TFR” relates to the amounts that employees in Italy are entitled to receive when they leave the company and is calculated based on the period of employment and the taxable earnings of each employee. Under certain conditions the entitlement may be partially advanced to an employee during the employee’s working life.
The Italian legislation regarding this scheme was amended by Law 296 of 27 December 2006 and subsequent decrees and regulations issued in the first part of 2007. Under these amendments, companies with at least 50 employees are obliged to transfer the TFR to the “Treasury fund” managed by the Italian state-owned social security body (“INPS”) or to supplementary pension funds. Prior to the amendments, accruing TFR for employees of all Italian companies could be managed by the company itself. Consequently, the Italian companies’ obligation to INPS and the contributions to supplementary pension funds take the form, under IAS 19 revised, of “Defined contribution plans” whereas the amounts recorded in the provision for employee severance pay retain the nature of “Defined benefit plans”. Accordingly, the provision for employee severance indemnity in Italy consists of the residual obligation for TFR until December 31, 2006. This is an unfunded defined benefit plan as the benefits have already been almost entirely earned, with the sole exception of future revaluations. Since 2007 the scheme has been classified as a defined contribution plan, and the Group recognizes the associated cost, being the required contributions to the pension funds, over the period in which the employee renders service.
Jubilee benefits
The Jubilee benefits scheme is applicable to companies incorporated in Germany. Upon retirement, employees are eligible to receive a sum payment depending on the number of years of service within the Group.
Other post-employment plans
Other post-employment plan of the Group are “Beneficios por Retiro, Prima de Antigüedad y Beneficios por Terminación” for Mexican companies and severance payment provision for Slovak companies.
F-56
Defined benefits obligation
The Group’s liabilities for employee benefits are as follows:
|
|
Trattamento |
|
|
Jubilee |
|
|
Beneficio |
|
|
Severance |
|
|
Total |
|
|||||
|
|
(EUR thousand) |
|
|||||||||||||||||
At January 1, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Interest cost |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
||||
Current service cost |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Benefits paid |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Actuarial gains and losses |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Exchange rate differences |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||
At December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Recognized in the consolidated income statement |
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
||||
Recognized in the other comprehensive income |
|
|
( |
) |
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
At January 1, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Acquisition of Perugini S.r.l. |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Interest cost |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Current service cost |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Benefits paid |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Actuarial gains and losses |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
||||
Exchange rate differences |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||
At December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Recognized in the consolidated income statement |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Recognized in the other comprehensive income |
|
|
|
|
|
— |
|
|
|
( |
) |
|
|
|
|
|
|
|||
A major assumption taken into account in the valuation of pension and other post-employment benefit obligations is the discount rate. In accordance with IAS 19 – Employee Benefits, the rates were determined by currency areas and by reference to the return on high-quality private bonds with a maturity equal to the term of the plans or the return on government bonds when the private market has insufficient liquidity. The return on plan assets is determined based on the allocation of the assets and the discount rates used.
The principal assumptions used for determining the obligations under the plan described are as follows:
At December 31, 2023
|
|
Severance indemnity |
|
|||||||||||||
|
|
Italy |
|
|
Germany |
|
|
Mexico |
|
|
Slovakia |
|
||||
Discount Rate % |
|
|
% |
|
|
% |
|
|
% |
|
|
% |
||||
Future salary increase % |
|
|
% |
|
|
— |
|
|
|
% |
|
|
% |
|||
Inflation rate % |
|
|
% |
|
|
— |
|
|
|
% |
|
|
— |
|
||
At December 31, 2022
|
|
Severance indemnity |
|
|||||||||||||
|
|
Italy |
|
|
Germany |
|
|
Mexico |
|
|
Slovakia |
|
||||
Discount Rate % |
|
|
% |
|
|
% |
|
|
% |
|
|
% |
||||
Future salary increase % |
|
|
% |
|
|
— |
|
|
|
% |
|
|
% |
|||
Inflation rate % |
|
|
% |
|
|
— |
|
|
|
% |
|
|
— |
|
||
The discount rates used for the measurement of the pension plan obligation (including Italian TFR obligation) are based on yields of high-quality fixed income securities for which the timing and amounts of payments match the timing and amounts
F-57
of the projected benefit payments. The main variation is due to Italian TFR, whose average duration is approximately
A quantitative sensitivity analysis for significant assumptions impacting defined benefits obligation as at December 31, 2023 and December 31, 2022 is reported as follows:
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
Turnover rate +1,00% |
|
|
|
|
|
|
||
Turnover rate -1,00% |
|
|
( |
) |
|
|
( |
) |
Inflation rate +0,25% |
|
|
|
|
|
|
||
Inflation rate -0,25% |
|
|
( |
) |
|
|
( |
) |
Annual discount rate +0,25% |
|
|
( |
) |
|
|
( |
) |
Annual discount rate -0,25% |
|
|
|
|
|
|
||
The above sensitivity analysis on TFR is based on reasonable changes in key assumptions occurring at the end of the reporting period, keeping all other assumptions constant.
Such analysis may not be representative of an actual change in the defined benefit obligation as it is unlikely that changes in assumptions would occur in isolation from one another.
Long-term incentive plan
In order to align the interests of management with those of the shareholders, the Group established a medium/ long-term incentive plan linking remuneration and performance. The Board of Directors approved a compensation plan called the “Long-term Incentive plan” for 2020-2023. The four-year plan included a selected number of top management and/ or key people and was based on achieving certain key performance indicators under the Group's long-term industrial plan targets.
On April 11, 2022 the Board of Directors approved an amendment to the "Restricted Stock Grant Plan Stevanato Group S.p.A. 2021-2027", in order to allow the entry of new beneficiaries in 2022, previously involved in the compensation plan called "Long Term Incentive 2020-2023". In accordance with specific rules, existing and new beneficiaries relating to the first vesting period coexisted during 2022 under the "Restricted Stock Grant Plan Stevanato Group S.p.A. 2021-2027". Through such amendment, beneficiaries of the Long Term Incentive 2020-2023 were offered the possibility of becoming beneficiaries of the "Restricted Stock Grant Plan Stevanato Group S.p.A. 2021-2027" in 2022 by using the rights deriving from the participation in the Long-Term Incentive 2020-2023 in the meantime accrued to them, but providing, that the free of charge transfer of the property of a certain number of Stevanato Group S.p.A. shares should have been done after the end of the First Vesting Period, after having verified the actual achievement of the Performance Objectives (in terms of consolidated revenue and EBITDA) set for such vesting period and the continuation of the employment relationship.
The letters of assignment of shares were issued to beneficiaries on May 12, 2022 and from that date the amendment of the incentive plans produced its accounting effects for those beneficiaries who accepted the incentive plan modification.
F-58
The Group’s liability for the Long-term Incentive plan is as follows:
|
|
Long Term Incentive Plan 2020-2023 |
|
|
|
|
(EUR thousand) |
|
|
At January 1, 2022 |
|
|
|
|
Interest cost |
|
|
|
|
Current service cost |
|
|
( |
) |
Actuarial gains and losses * |
|
|
( |
) |
At December 31, 2022 |
|
|
|
|
Benefits paid |
|
|
( |
) |
At December 31, 2023 |
|
|
— |
|
*According to IAS 19, actuarial gains and losses are recognized in profit or loss
Restricted Stock Grant Plan 2021-2027
At the Shareholders’ Meeting of Stevanato Group held on March 4, 2021, a share-based incentive plan, referred to as the “Restricted Stock Grant Plan 2021-2027”, was approved. This plan included individuals who play a strategic role in the Group related to the economic and strategic development of the Group and aligns their interests to those of the shareholders and other stakeholders of the Company, during the period between January 1, 2021 and December 31, 2026.
At the beginning of the vesting period, a certain number of Stevanato Group ordinary shares – linked with the achievement of specific targets in terms of consolidated revenue and EBITDA within the end of the Vesting Period – was assigned free of charge to the initial beneficiaries. EBITDA is defined as net profit before income taxes, finance income, finance expense, depreciation and amortization. The assigned shares were subject to the prohibition to sell and to the selling commitment in accordance with a one-year lock-up period.
The transfer of ownership of the shares was finalized after each initial beneficiary had signed an agreement which binded the beneficiaries to re-sell to Stevanato Group, fully or partially, the Shares assigned to them in case the targets provided for the vesting period in relation to which the shares were assigned should not have been totally or partially achieved. A similar obligation was provided if, within the end of the vesting period, the employment relationship would have terminated.
In the event of over-performances related to the Key Indicators of Performance, initial beneficiaries were granted, free of charge, an additional number of Stevanato Group shares related to the vesting period in which the targets were exceeded and the additional shares assigned would have been subject to the time-limited prohibition to sell.
On June 3, 2021 a total of n.
The fair value measurement of the stock grant plan for the initial beneficiaries consists of the following components:
- a first IAS 19 component linked to the cash settlement of the amount equal to the consideration already determined at which Stevanato Group S.p.A. would have repurchased the shares in the cases provided for by the plan regulation. This component was immediately vested at the time of the assignment of the shares. It generated expenses counterbalanced with the employee benefits liability;
F-59
- a second IFRS 2 component related to the benefit associated with the value of the stock. It is valued as stock option with a strike price equal to the value corresponding to the consideration the employees gave up in cash when the stock option was exercised. It generated expenses counterbalanced in a dedicated equity reserve among "other reserves".
On May 12, 2022, Stevanato Group S.p.A. sent, to the new beneficiaries of shares, a letter granting them the right to obtain the transfer free of charge of a certain number of shares if the performance targets, in terms of consolidated revenue and EBITDA provided for the vesting period in relation to which the shares were assigned, would have been achieved. New beneficiaries are individuals who play a strategic role in the Group, including its economic and strategic development, and the above right to transfer shares (subject to certain conditions) aligned their interests to those of the shareholders and other stakeholders of the Company, during the period between January 1, 2021 and December 31, 2022.
The right for each of the new beneficiaries to receive shares was conditional upon the verification by Stevanato Group's Board of Directors of the degree of achievement of each of the performance targets after the end of the first vesting period. On the basis of this assessment the number of shares indicated in the letter of attribution of rights could have been reduced based on the degree of target achievement.
At August 30, 2023 and at September 7, 2023 the ownership of the
The following table summarizes the IAS 19 components of the obligation expense recognized in the statement of profit or loss and amounts recognized in the statement of financial position:
|
|
Stock grant plan 2021-2027 |
|
|
|
|
(EUR thousand) |
|
|
At January 1, 2022 |
|
|
|
|
Current service cost |
|
|
|
|
At December 31, 2022 |
|
|
|
|
Reclassified in Equity Reserve for share-based incentive plans |
|
|
( |
) |
At December 31, 2023 |
|
|
|
|
Other share-based compensation
At December 31, 2022, the Group recognized a liability for other share-based compensation amounting to EUR
29. Share-based compensation
On December 15, 2022, the Board of Directors approved a Long-Term Incentive Plan including
On January 3, 2023 the beneficiaries involved in the new Restricted Shares Plan 2023-2027 and Performance Shares Plan 2023-2027 received a letter that granted them the right to obtain the transfer free of charge of a certain number of shares if the underlying conditions are met. The Restricted Shares Plan forms part of Stevanato Group’s long-term remuneration policy wherein Restricted Share Units represent, for the first vesting period (January 2023 - December 2025),
The granting of awards under the Restricted Shares Plan, for each vesting period, is subject to and dependent on the satisfaction of the following presence condition: shares shall not vest unless, at the end of the presence period related to each installment -
F-60
unless otherwise agreed by the Chief Executive Officer. In particular, the presence period is differentiated in coherence with the vesting schedule and coincides with the period between the grant of rights date and each installment-vesting schedule.
The right to the award of shares under the Performance Shares Plan, for each vesting period -
I.
II.
The performance target level, minimum target, overachievement target and maximum target of each performance criterion, for each vesting period, were communicated to the beneficiaries with the grant letter.
The fair values of the Restricted Share Unit (RSU) and Performance Share Unit (PSU) awards were measured using the share price on the grant date adjusted for expected annual dividend yield of
|
|
Long Term Incentive Plan 2023 -2027 |
|
|
Granted in 2023 |
Performance Share Units (PSUs) |
|
EUR |
Restricted Share Units (RSUs) - I Installment |
|
EUR |
Restricted Share Units (RSUs) - II Installment |
|
EUR |
Restricted Share Units (RSUs) - III Installment |
|
EUR |
Changes to the unvested number of PSU and RSU awards under all equity incentive plans of the Group are as follows:
number of shares |
|
Outstanding PSUs |
|
|
Outstanding RSUs |
|
||
At January 1, 2023 |
|
|
— |
|
|
|
— |
|
Granted (*) |
|
|
|
|
|
|
||
Forfeited |
|
|
( |
) |
|
|
( |
) |
At December 31, 2023 |
|
|
|
|
|
|
||
For the year ended December 31, 2023, the Company recognized EUR
F-61
30. Provisions
The balances as of December 31, 2023 are detailed below:
|
|
Provision for |
|
|
Decommissioning |
|
|
Provision for |
|
|
Provision for |
|
|
Total |
|
|||||
|
|
(EUR thousand) |
|
|||||||||||||||||
At January 1, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Accrued during the period |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Utilization |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Releases |
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
||
Exchange rate differences |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
( |
) |
||
At December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Current |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Non-current |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
Provision for |
|
|
Decommissioning |
|
|
Provision for |
|
|
Provision for |
|
|
Total |
|
|||||
|
|
(EUR thousand) |
|
|||||||||||||||||
At January 1, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Accrued during the period |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Utilization |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Releases |
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
||
Exchange rate differences |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
At December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Current |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Non-current |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
The warranty provision represents the best estimate of commitments given by the Group for contractual, legal, or constructive obligations arising from product warranties given for a specified period of time. Such provisions are recognized on shipment of goods to customers. The warranty provision is estimated on the basis of the Group’s past experience and contractual terms. Related costs are recognized within cost of sales.
The provision for legal proceeding and sundry risks represents management’s best estimate of the expenditures expected to be required to settle or otherwise resolve legal proceeding and disputes. At December 31, 2023 the Group accrued EUR
At December 31, 2023 provision for legal and sundry risks also include accruals in connection with taxation related to personnel severance amounting to EUR
31. Other non-current liabilities
Other non-current liabilities at December 31, 2023 and December 31, 2022 amounted to EUR
F-62
a partial payment for installing machinery in Fishers, Indiana, to help strengthen domestic capabilities in the U.S. for national defense readiness and preparedness programs for current and future public health emergencies; and (ii) an advance payment from the city of Fishers for certain costs at the site of EUR
32. Trade payables and other current liabilities
Trade payables and other current liabilities are detailed as follows:
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
Trade payables |
|
|
|
|
|
|
||
Payables to social security institutions |
|
|
|
|
|
|
||
Payables to personnel |
|
|
|
|
|
|
||
VAT payables |
|
|
|
|
|
|
||
Other tax payables |
|
|
|
|
|
|
||
Deferred income and prepayments |
|
|
|
|
|
|
||
Allowance for future expected customer returns |
|
|
|
|
|
|
||
Other |
|
|
|
|
|
|
||
Total trade payables and other current liabilities |
|
|
|
|
|
|
||
The book value of trade payables is approximately equal to their fair value. Trade payables are non-interest bearing and are normally settled on
Other current liabilities include customer returns that reflect the improved estimate of expected liabilities related to future expected returns regarding revenue recognized in the current or in previous years, estimated on the basis of past experience.
In 2018 the Group launched the “Confirming program”, a web-based and pay-per-use Supply Chain Finance solution, that allows Group suppliers to anticipate their receivables. The main benefits for the Group are an improvement of supply chain financial stability and a simplification in payment management cycle. Under such program, the suppliers can elect on an invoice-by-invoice basis to receive a discounted early payment from the relationship bank rather than being paid in line with the agreed payment terms. If the option is taken, the Group's liability is assigned by the supplier to be due to the relationship bank rather than the supplier. The value of the liability payable by the Group remains unchanged. At December 31, 2023 the total amount of accounting payables related to the Confirming program equaled to EUR
33. Contract liabilities and advances from customers
Contract liabilities and advances from customers are as follows:
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
Contract Liabilities |
|
|
|
|
|
|
||
Advances from customers |
|
|
|
|
|
|
||
Total contract liabilities and advances from customers |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Current |
|
|
|
|
|
|
||
Non-current |
|
|
|
|
|
|
||
F-63
Contract liabilities relate to ongoing customer-specific construction contracts of the Engineering Segment and of the In-vitro diagnostic business, which is part of the Biopharmaceutical and Diagnostic Solutions Segment. The Group has contract liabilities of EUR
Advances from customers relate to sales whose revenue are recognized at point in time.
34. Leases
The Group has lease contracts for various items of plant, machinery, vehicles and other equipment used in its operations. Leases of plant and machinery generally have lease terms between
The Group also has certain leases of machinery, industrial equipment and vehicles with lease terms of
Movements in the leased right of use assets in 2023 are shown below:
|
|
Buildings |
|
|
Plant and |
|
|
Industrial |
|
|
Other |
|
|
Total |
|
|||||
|
|
(EUR thousand) |
|
|||||||||||||||||
Cost |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
At January 1, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Additions |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|||
Disposals |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Exchange rate differences |
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
||||
At December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Acquisition of Perugini S.r.l. |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|||
Additions |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|||
Disposals |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Exchange rate differences |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
|
|
|
( |
) |
|
At December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Depreciation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
At January 1, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Depreciation charge for the year |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Disposals |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Exchange rate differences |
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
||||
At December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Acquisition of Perugini S.r.l. |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|||
Depreciation charge for the year |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Disposals |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Exchange rate differences |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
At December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Net book value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
At December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
At December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
F-64
Set out below are the carrying amounts of lease liabilities and the movements during the period:
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
At January 1 |
|
|
|
|
|
|
||
Additions |
|
|
|
|
|
|
||
Acquisition of Perugini S.r.l. |
|
|
|
|
|
— |
|
|
Accretion of interest |
|
|
|
|
|
|
||
Payments |
|
|
( |
) |
|
|
( |
) |
Early terminated contracts |
|
|
( |
) |
|
|
( |
) |
Exchange rate difference |
|
|
( |
) |
|
|
|
|
At December 31 |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Current |
|
|
|
|
|
|
||
Non-current |
|
|
|
|
|
|
||
The following are the amounts recognized in profit or loss:
|
|
For the year ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
(EUR thousand) |
|
|||||||||
Depreciation expense of right of use assets |
|
|
|
|
|
|
|
|
|
|||
Interest expense on lease liabilities |
|
|
|
|
|
|
|
|
|
|||
Expense relating to short-term leases |
|
|
|
|
|
|
|
|
|
|||
Expense relating to leases of low-value assets |
|
|
|
|
|
|
|
|
|
|||
Total amount recognized in profit or loss |
|
|
|
|
|
|
|
|
|
|||
At December 31, 2023 the Group signed (i) a binding offer for a
35. Subsidiaries with non-controlling interest
The Stevanato Group comprises the following subsidiaries with non-controlling interest:
|
|
|
|
At December 31, |
|
|
At December 31, |
|
||
Name |
|
Country |
|
2023 |
|
|
2022 |
|
||
|
|
|
|
|
|
|
|
|
||
Ompi of Japan Co., Ltd. |
|
|
|
% |
|
|
% |
|||
Medical Glass a.s. |
|
|
|
% |
|
|
% |
|||
|
|
|
|
|
|
|
|
|
||
|
|
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
|
|
2023 |
|
|
2022 |
|
||
|
|
|
|
(EUR thousand) |
|
|||||
|
|
|
|
|
|
|
|
|
||
Proportion of equity interest held by non-controlling interests: |
|
|
|
|
|
|
|
|
||
Ompi of Japan Co., Ltd. |
|
|
|
|
— |
|
|
|
|
|
Medical Glass a.s. |
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Profit allocated to non-controlling interest: |
|
|
|
|
|
|
|
|
||
Ompi of Japan Co., Ltd. |
|
|
|
|
( |
) |
|
|
( |
) |
Medical Glass a.s. |
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
F-65
Changes in non-controlling interests are shown in the consolidated statement of changes in equity.
Effective July 31, 2023, Stevanato Group entered into an agreement with Winckler & Co. Ltd, SE Holdings Co. Ltd and Ompi of Japan Co., Ltd., to purchase the remaining minority equity interests in Ompi of Japan Co., Ltd. and to terminate the JV agreement with SE Holdings Co. Ltd. The consideration paid for purchasing the non-controlling interests in Ompi of Japan Co., Ltd. amounted to EUR
36. Related party disclosures
According to IAS 24, the related parties of the Group are entities and individuals capable of exercising control, joint control or significant influence over the Group and its subsidiaries, companies belonging to the Stevanato Group S.p.A., the controlling company Stevanato Holding S.r.l. and associates. In addition, members of Stevanato Group’s Board of Directors and executives with strategic responsibilities and their families are also considered related parties. The Group carries out transactions with related parties on commercial terms that are normal in the respective markets, considering the characteristics of the goods or services involved.
Note 4 provides information about the Group’s structure, including details of the subsidiaries and the holding company.
Transactions with related parties refer to:
F-66
The amounts of transactions with related parties recognized in the consolidated income statement and the related assets and liabilities are as follows:
For the year ended and at December 31, 2023
|
|
Revenue |
|
|
Costs* |
|
||
|
|
(EUR thousand) |
|
|||||
|
|
|
|
|
|
|
||
Other related parties |
|
|
|
|
|
|
||
Winckler & Co. Ltd. |
|
|
— |
|
|
|
|
|
Società Agricola Stella S.r.l. |
|
|
— |
|
|
|
|
|
SFEM Italia S.r.l. |
|
|
— |
|
|
|
|
|
E & FKH Ejendomme ApS |
|
|
— |
|
|
|
|
|
Studio Legale Spinazzi Azzarita Troi |
|
|
— |
|
|
|
|
|
Fondazione Stevanato |
|
|
— |
|
|
|
|
|
C.T.S. Studio AS |
|
|
— |
|
|
|
|
|
Incog BioPharma Services Inc |
|
|
|
|
|
— |
|
|
* Costs include cost of sales, selling, general administrative costs and other expenses net
|
|
Trade |
|
|
Trade |
|
|
Other Assets |
|
|
Other |
|
||||
|
|
(EUR thousand) |
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Parent company: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Stevanato Holding S.r.l. |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Other related parties |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Società Agricola Stella S.r.l. |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
Studio Legale Spinazzi Azzarita Troi |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
C.T.S. Studio AS |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
SIT Manufacturing |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
Incog BioPharma Services Inc |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Loan from/to related parties
For the year ended and at December 31, 2023
|
|
Interest |
|
|
Interest |
|
|
Financial |
|
|||
|
|
(EUR thousand) |
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|||
Other related parties |
|
|
|
|
|
|
|
|
|
|||
SE Holdings Co.Ltd. |
|
|
— |
|
|
|
|
|
|
— |
|
|
F-67
For the year ended and at December 31, 2022
|
|
Revenue |
|
|
Costs* |
|
||
|
|
(EUR thousand) |
|
|||||
|
|
|
|
|
|
|
||
Other related parties |
|
|
|
|
|
|
||
Winckler & Co. Ltd. |
|
|
— |
|
|
|
|
|
Società Agricola Stella S.r.l. |
|
|
— |
|
|
|
|
|
SFEM Italia S.r.l. |
|
|
— |
|
|
|
|
|
E & FKH Ejendomme ApS |
|
|
— |
|
|
|
|
|
Piovesan Barbara |
|
|
— |
|
|
|
|
|
Studio Legale Spinazzi Azzarita Troi |
|
|
— |
|
|
|
|
|
Fondazione Stevanato |
|
|
— |
|
|
|
|
|
C.T.S. Studio AS |
|
|
— |
|
|
|
|
|
Incog BioPharma Services Inc |
|
|
|
|
|
— |
|
|
|
|
Trade |
|
|
Trade |
|
|
Other |
|
|
Other |
|
||||
|
|
(EUR thousand) |
|
|||||||||||||
Other related parties |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Winckler & Co. Ltd. |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
Società Agricola Stella S.r.l. |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
SFEM Italia S.r.l. |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
Studio Legale Spinazzi Azzarita Troi |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
C.T.S. Studio AS |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
Incog BioPharma Services Inc |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
* Costs include cost of sales, selling, general administrative costs and other expenses net
Loan from/to related parties
For the year ended and at December 31, 2022
|
|
Interest |
|
|
Interest |
|
|
Financial |
|
|||
|
|
(EUR thousand) |
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|||
Other related parties |
|
|
|
|
|
|
|
|
|
|||
SE Holdings Co.Ltd. |
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|||
Key management personnel of the Group |
|
|
|
|
|
|
|
|
|
|||
Directors and Key Managers |
|
|
|
|
|
— |
|
|
|
— |
|
|
F-68
For the year ended December 31, 2021
|
|
Revenue |
|
|
Costs* |
|
||
|
|
(EUR thousand) |
|
|||||
|
|
|
|
|
|
|
||
Parent company |
|
|
|
|
|
|
||
Stevanato Holding S.r.l. |
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
||
Associate companies |
|
|
|
|
|
|
||
Swissfillon AG |
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
||
Other related parties |
|
|
|
|
|
|
||
Winckler & Co. Ltd. |
|
|
— |
|
|
|
|
|
Società Agricola Stella S.r.l. |
|
|
— |
|
|
|
|
|
SFEM Italia S.r.l. |
|
|
— |
|
|
|
|
|
MJB Consultants LLC |
|
|
— |
|
|
|
|
|
Progenitor Capital Partners LLC |
|
|
— |
|
|
|
|
|
E & FKH Ejendomme ApS |
|
|
— |
|
|
|
|
|
Piovesan Barbara |
|
|
— |
|
|
|
|
|
Studio Legale Spinazzi Azzarita Troi |
|
|
— |
|
|
|
|
|
Federici William |
|
|
— |
|
|
|
|
|
Fondazione Stevanato |
|
|
— |
|
|
|
|
|
C.T.S. Studio AS |
|
|
— |
|
|
|
|
|
Incog BioPharma Services Inc |
|
|
|
|
|
— |
|
|
* Costs include cost of sales, selling, general administrative costs and other expenses net
Loan from/to related parties
For the year ended December 31, 2021
|
|
Interest |
|
|
Interest |
|
||
|
|
(EUR thousand) |
|
|||||
|
|
|
|
|
|
|
||
Associate companies |
|
|
|
|
|
|
||
Swissfillon AG |
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
||
Other related parties |
|
|
|
|
|
|
||
SE Holdings Co.Ltd. |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
||
Key management personnel of the Group |
|
|
|
|
|
|
||
Directors and Key Managers |
|
|
|
|
|
— |
|
|
Emoluments to Directors and Key Management
The fees of the Directors of Stevanato Group S.p.A. are as follows:
For the year ended December 31, 2023
|
|
Fixed remuneration |
|
|
Pension |
|
|
Share based |
|
|
Total |
|
||||||||
|
|
Annual |
|
|
Fringe |
|
|
expense (2) |
|
|
compensation (3) |
|
|
remuneration |
|
|||||
|
|
fee |
|
|
benefits (1) |
|
|
|
|
|
|
|
|
|
|
|||||
|
|
(EUR thousand) |
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Total Directors |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
F-69
(1) Fringe benefits related to car and insurance benefits
(2) Pensions expense related to "Trattamento Fine Mandato" accrued in the year
(3) Shares granted to board members
For the year ended December 31, 2022
|
|
Fixed remuneration |
|
|
Pension |
|
|
Share based |
|
|
Total |
|
||||||||
|
|
Annual |
|
|
Fringe |
|
|
expense (2) |
|
|
compensation (3) |
|
|
remuneration |
|
|||||
|
|
fee |
|
|
benefits (1) |
|
|
|
|
|
|
|
|
|
|
|||||
|
|
(EUR thousand) |
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Total Directors |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
(1) Fringe benefits related to car and insurance benefits
(2) Pensions expense related to "Trattamento Fine Mandato" accrued in the year
(3) Shares granted to board members
For the year ended December 31, 2021
|
|
Fixed remuneration |
|
|
Pension |
|
|
Long Term |
|
|
Share based |
|
|
Total |
|
|||||||||
|
|
Annual |
|
|
Fringe |
|
|
expense(2) |
|
|
Benefits(3) |
|
|
compensation (4) |
|
|
remuneration |
|
||||||
|
|
fee |
|
|
benefits (1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
(EUR thousand) |
|
|||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Total Directors |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
( |
) |
||||
(1) Fringe benefits related to car and insurance benefits
(2) Pensions expense related to "Trattamento Fine Mandato" accrued in the year
(3) Long term benefits related to cash settled awards early terminated in 2021
(4) Shares granted to board members
The aggregate compensation for members of the Senior Management Team (excluding the Chairman and including the CEO) is as follows:
For the year ended December 31, 2023
|
|
Fixed remuneration |
|
|
Variable |
|
|
Pension |
|
|
Share based |
|
|
Total |
|
|||||||||
|
|
Annual |
|
|
Fringe |
|
|
remuneration(2) |
|
|
expense (3) |
|
|
compensation(4) |
|
|
remuneration |
|
||||||
|
|
fee |
|
|
benefits(1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
(EUR thousand) |
|
|||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Total Other Key Management |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
(1) Fringe benefits related to car and insurance benefits
(2) Variable remuneration related to MBO
(3) Pensions expense related to "Trattamento Fine Rapporto" accrued in the year
(4) Shares granted under stock grant plan 2023-2027 and other share-based incentive plans
For the year ended December 31, 2022
|
|
Fixed remuneration |
|
|
Variable |
|
|
Pension |
|
|
Share based |
|
|
Total |
|
|||||||||
|
|
Annual |
|
|
Fringe |
|
|
remuneration(2) |
|
|
expense(3) |
|
|
compensation(5) |
|
|
remuneration |
|
||||||
|
|
fee |
|
|
benefits(1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
(EUR thousand) |
|
|||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Total Other Key Management |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
F-70
(1) Fringe benefits related to car and insurance benefits
(2) Variable remuneration related to MBO and LTI. With regard to variable compensation, key managers' performance is measured not only by financial indicators, such as revenue and EBITDA margin, but also by non-financial indicators such as (i) environment: programs in line with carbon neutrality, (ii) gender balance in senior position, (iii) quality mindset and performances and (iv) values and guiding principles.
(3) Pensions expense related to "Trattamento Fine Rapporto" accrued in the year
(4) Shares granted under stock grant plan 2021-2027 and other share-based incentive plans
For the year ended December 31, 2021
|
|
Fixed remuneration |
|
|
Variable |
|
|
Pension |
|
|
Long Term |
|
|
Share based |
|
|
Total |
|
||||||||||
|
|
Annual |
|
|
Fringe |
|
|
remuneration(2) |
|
|
expense(3) |
|
|
Benefits(4) |
|
|
compensation(5) |
|
|
remuneration |
|
|||||||
|
|
fee |
|
|
benefits(1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
(EUR thousand) |
|
|||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Total Other Key Management |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
( |
) |
|||||
(1) Fringe benefits related to car and insurance benefits
(2) Variable remuneration related to MBO and LTI
(3) Pensions expense related to "Trattamento Fine Rapporto" accrued in the year
(4) Long term benefits related to cash settled awards early terminated in 2021
(5) Share-based compensation awarded under stock grant plan 2021-2027
37. Commitments and contingencies
Commitments, guarantees and contingent liabilities can be described as follows:
|
|
At December 31, |
|
|
At December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
(EUR thousand) |
|
|||||
Guarantees |
|
|
|
|
|
|
||
of which secured |
|
|
|
|
|
|
||
Total Guarantees |
|
|
|
|
|
|
||
At December 31, 2023 the main commitments and risks assumed by the Stevanato Group are as follows:
Secured guarantees for EUR
The guarantees provided by credit institutions and insurance companies on behalf of Group companies in favor of third parties amount to EUR
Other residual guarantees with individual low value amounts, are in aggregate EUR
From time to time, in the ordinary course of business, the Group enters into various arrangements with key third party suppliers. A limited number of these arrangements contain unconditional purchase obligations to purchase a fixed or minimum quantity of goods with fixed and determinable price provisions. At December 31, 2023, the Group is in compliance with the contract obligations.
F-71
38. Qualitative and quantitative information of financial risks
The Group is exposed to the following financial risks connected with its operations:
These risks could significantly affect the Group’s financial position, results of operations and cash flows, and for this reason the Group identifies and monitors these risks, in order to detect potential negative effects in advance and take the necessary action to mitigate them, primarily through its operating and financing activities and if required, through the use of derivative financial instruments.
The following section provides qualitative and quantitative disclosures on the effect that these risks may have upon the Group. The quantitative data reported in the following section does not have any predictive value.
Financial market risks
Due to the nature of the Group’s business, the Group is exposed to a variety of market risks, including foreign currency exchange rate risk, interest rate risk and commodity risk.
The Group’s exposure to foreign currency exchange rate risk arises from our global footprint (both in terms of productions and commercialization), as in some cases we sell our products in the currencies of the destination markets, which may differ from the currency of the countries in which the Group operates.
The Group’s exposure to interest rate risk arises from the need to fund certain activities and the possibility to deploy surplus funds. Changes in market interest rates may have the effect of either increasing or decreasing the Group’s net profit/ (loss), thereby indirectly affecting the costs and returns of financing and investing transactions.
These risks could significantly affect the Group’s financial position, results of operations and cash flows, and for this reason they are identified and monitored, in order to detect potential negative effects in advance and take the necessary actions to mitigate them.
The Group has in place various risk management policies, which primarily relate to foreign exchange, interest rate, commodity price and liquidity risks.
In particular, to manage foreign exchange rate risk, the Group has adopted a hedging policy, approved by the Board of Directors of Stevanato Group S.p.A.. Hedging activities are mainly executed at central level, based on the information provided by the reporting system and utilizing instruments and policies conforming to IFRS. Hedging is undertaken to ensure protection in case an entity has transactions in currencies other than the one in which it primarily does business, taking account also of budgeted future revenue/ costs. Despite hedging operations, sudden movements in exchange rates or erroneous estimates may result in a negative impact, although limited, on Group results.
Information on foreign currency exchange rate risk
The Group is exposed to risk resulting from fluctuations in foreign currency exchange rates, which can affect its earnings and equity. In particular:
F-72
The Group monitors its main exposures with regard to translation exchange risk, whereby fluctuations in the exchange rates of a number of currencies against the consolidation currency may impact the consolidated financial statement values, although there was no specific hedging in this respect at the reporting date.
Exchange differences arising on the settlement of monetary items are recognized in the consolidated income statement within the net financial income/ (expense) line item.
The impact of foreign currency exchange rate differences recorded within financial income/ (expense) for the year ended December 31, 2023, except for those arising on financial instruments measured at fair value, amounted to a net loss of EUR
There have been no substantial changes in 2023 in the nature or structure of exposure to foreign currency exchange rate risk or in the Group’s hedging policies.
The Group actively hedges against economic-transactional risk; more specifically, forward and swap contracts, plain vanilla and collar options are used to manage the exposures. Such instruments are only partially designated as cash flow hedges and contracts are entered for a period consistent with the underlying transactions, generally from three to twelve months.
F-73
The Group is holding the following contracts:
At December 31, 2023
|
|
|
|
0 to 6 |
|
6 to 9 |
|
9 to 12 |
|
Total |
|
Carrying |
|
Line item in the |
|
|
(EUR thousand) |
||||||||||||
Notional amount (2) |
|
Forward |
|
|
— |
|
— |
|
|
|
Other current financial assets |
|||
Average forward rate (EUR/USD) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notional amount (1) |
|
Collar options |
|
|
— |
|
— |
|
|
|
Other current financial assets |
|||
Average forward rate (USD/MXN) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notional amount (2) |
|
Forward |
|
|
|
— |
|
|
|
Other current financial assets |
||||
Average forward rate (EUR/USD) |
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|
|
|
|
|
|
|
|
|
|
|
||
(1) Derivatives not designated as hedging instruments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(2) Derivatives designated as hedging instruments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2022
|
|
|
|
0 to 6 |
|
|
6 to 9 |
|
|
9 to 12 |
|
|
Total |
|
|
Carrying |
|
|
Line item in the |
|||||
|
|
(EUR thousand) |
||||||||||||||||||||||
Notional amount (1) |
|
Forward |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other current financial assets |
|||||
Average forward rate (EUR/USD) |
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
||||
Notional amount (2) |
|
Forward |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other current financial assets |
|||||
Average forward rate (EUR/USD) |
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
||||
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
(1) Derivatives not designated as hedging instruments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
(2) Derivatives designated as hedging instruments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Set out below is the impact of hedging on equity:
|
|
Cash Flow Hedge Reserve |
|
|
Cost of Hedging Reserve |
|
||
|
|
(EUR thousand) |
|
|||||
At January 1, 2022 |
|
|
— |
|
|
|
— |
|
Foreign exchange forward |
|
|
( |
) |
|
|
|
|
Tax effect |
|
|
|
|
|
( |
) |
|
At 31 December, 2022 |
|
|
( |
) |
|
|
|
|
Foreign exchange forward |
|
|
|
|
|
( |
) |
|
Tax effect |
|
|
( |
) |
|
|
|
|
At 31 December, 2023 |
|
|
( |
) |
|
|
|
|
F-74
The following table presents an analysis of sensitivity to a change in exchange rates for the currencies the Group is majorly exposed to. Such analysis does not consider the impact of forward currency contracts or collar options. With all other variables held constant, the Group’s marginality is affected as follows:
At December 31, 2023
Exchange rate sensitivity |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
Increase/decrease |
|
|
Effect on operating profit |
|
||||||||||
|
|
(EUR thousand) |
|
|||||||||||||
Euro |
|
|
% |
|
|
( |
)% |
|
|
( |
) |
|
|
|
||
US dollar |
|
|
% |
|
|
( |
)% |
|
|
( |
) |
|
|
|
||
|
|
|
% |
|
|
( |
)% |
|
|
( |
) |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Euro |
|
|
% |
|
|
( |
)% |
|
|
|
|
|
( |
) |
||
Mexican Pesos |
|
|
% |
|
|
( |
)% |
|
|
|
|
|
( |
) |
||
|
|
|
% |
|
|
( |
)% |
|
|
|
|
|
( |
) |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Euro |
|
|
% |
|
|
( |
)% |
|
|
( |
) |
|
|
|
||
China Renmimbi |
|
|
% |
|
|
( |
)% |
|
|
( |
) |
|
|
|
||
|
|
|
% |
|
|
( |
)% |
|
|
( |
) |
|
|
|
||
At December 31, 2022
Exchange rate sensitivity |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
Increase/decrease |
|
|
Effect on operating profit |
|
||||||||||
|
|
(EUR thousand) |
|
|||||||||||||
Euro |
|
|
% |
|
|
( |
)% |
|
|
( |
) |
|
|
|
||
US dollar |
|
|
% |
|
|
( |
)% |
|
|
( |
) |
|
|
|
||
|
|
|
% |
|
|
( |
)% |
|
|
( |
) |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Euro |
|
|
% |
|
|
( |
)% |
|
|
|
|
|
( |
) |
||
Mexican Pesos |
|
|
% |
|
|
( |
)% |
|
|
|
|
|
( |
) |
||
|
|
|
% |
|
|
( |
)% |
|
|
|
|
|
( |
) |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Euro |
|
|
% |
|
|
( |
)% |
|
|
( |
) |
|
|
|
||
China Renmimbi |
|
|
% |
|
|
( |
)% |
|
|
( |
) |
|
|
|
||
|
|
|
% |
|
|
( |
)% |
|
|
( |
) |
|
|
|
||
Information on interest rate risk
This risk stems from variable rate loans, for which sudden or significant interest rate fluctuations may have a negative impact on economic results. The monitoring of this risk is carried out at corporate level and utilizing similar structures as those employed for the management of currency risks. The Group has hedges in place against interest rate risk, covering EUR
The Group’s most significant floating rate financial assets at December 31, 2023 are cash and cash equivalents.
F-75
The financial liabilities composition and the impact of the hedging instrument on the statement of financial position at December 31, 2023 and December 31, 2022 are as follows:
At December 31, 2023
|
|
Fix Through derivatives |
|
|
FIX |
|
|
Floating |
|
|
Total |
|
|
Effect |
|
|
Total |
|
|
MtM |
|
|
Line item in |
|||||||
|
|
(EUR thousand) |
|
|
|
|||||||||||||||||||||||||
Bank loans |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|||||||
Bank overdrafts |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
||||
Financial payables for share acquisition |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
||||
Notes |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
|
|
|
— |
|
|
||||
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Percentage of Total |
|
|
% |
|
|
% |
|
|
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
At December 31, 2022
|
|
Fix Through derivatives |
|
|
FIX |
|
|
Floating |
|
|
Total |
|
|
Effect |
|
|
Total |
|
|
MtM |
|
|
Line item in |
|||||||
|
|
(EUR thousand) |
|
|
|
|||||||||||||||||||||||||
Bank loans |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|||||||
Bank overdrafts |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
||||
Financial payables for share acquisition |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
Financial liabilities due to related parties |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
||||
Financial liabilities due to other lenders |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|||
Notes |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
|
|
|
— |
|
|
||||
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Percentage of Total |
|
|
% |
|
|
% |
|
|
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
F-76
The Group is holding the following hedging contracts (Interest Rate Swaps):
Line item in the statement of financial position |
|
Contract notional |
|
|
Currency |
|
Carrying amount at December 31, 2023 |
|
|
Carrying amount at December 31, 2022 |
|
|||
|
|
(EUR thousand) |
|
|||||||||||
Non-current financial assets |
|
|
|
|
EUR |
|
|
|
|
|
|
|||
Current financial assets |
|
|
|
|
EUR |
|
|
|
|
|
|
|||
Non-current financial liabilities |
|
|
|
|
EUR |
|
|
( |
) |
|
|
— |
|
|
The risk arising from net investment in foreign subsidiaries is monitored; no active hedging is currently being performed. With regard to commodity risk, the Group enters into fixed-price contracts for certain utilities.
Set out below is the impact of hedging on equity:
|
|
Cash Flow Hedge Reserve |
|
|
|
|
(EUR thousand) |
|
|
At January 1, 2022 |
|
|
|
|
Interest Rate Swap |
|
|
( |
) |
Tax effect |
|
|
|
|
At December 31, 2022 |
|
|
( |
) |
Interest Rate Swap |
|
|
|
|
Tax effect |
|
|
( |
) |
At December 31, 2023 |
|
|
( |
) |
The following table presents an analysis of sensitivity to a change in interest rates on the portion of loans and borrowings affected. With all other variables held constant, the Group’s marginality is affected as follows:
At December 31, 2023
Interest rate sensitivity |
|
|
|
|
|
|
|
|
|
|
||
|
|
Increase/decrease |
|
Effect on profit |
|
|||||||
|
|
(EUR thousand) |
|
|||||||||
|
|
+ |
|
- |
|
|
( |
) |
|
|
|
|
|
|
+ |
|
- |
|
|
( |
) |
|
|
|
|
|
|
+ |
|
- |
|
|
( |
) |
|
|
|
|
At December 31, 2022
Interest rate sensitivity |
|
|
|
|
|
|
|
|
|
|
||
|
|
Increase/decrease |
|
Effect on profit |
|
|||||||
|
|
(EUR thousand) |
|
|||||||||
|
|
+ |
|
- |
|
|
— |
|
|
|
— |
|
|
|
+ |
|
- |
|
|
— |
|
|
|
— |
|
|
|
+ |
|
- |
|
|
— |
|
|
|
— |
|
Liquidity risk
Liquidity risk arises if the Group is unable to obtain the funds needed to carry out its operations under economic conditions. The main determinant of the Group’s liquidity position is the cash generated by or used in operating and investing activities.
F-77
From an operating point of view, the Group manages liquidity risk by monitoring cash flows and keeping an adequate level of funds at its disposal. The main funding operations and investments in cash and marketable securities of the Group are centrally managed or supervised by the treasury department with the aim of ensuring effective and efficient management of the Group’s liquidity. The Group undertakes medium/long term loans to fund medium/long term operations. The Group undertakes a series of activities centrally supervised with the purpose of optimizing the management of funds and reducing liquidity risk, such as:
Intercompany financing is conducted at arm’s length terms and normally involves the holding company. These measures currently sufficiently guarantee, at normal conditions and in the absence of extraordinary events, the degree of flexibility required by movements of working capital, investing activities and cash flows in general.
The Group believes that its total available liquidity (defined as cash and cash equivalents plus undrawn committed credit lines), and the potential access to additional capital through the equity markets or through additional loan or debt agreements, will enable us to satisfy the requirements of our investing activities and working capital needs, fulfill its obligations to repay its debt and ensure an appropriate level of operating and strategic flexibility for at least the next 12 months. However, there can be no assurance that the Group will be able to obtain additional capital, or at acceptable costs.
The following table summarizes the due dates of the Group’s financial and other liabilities at December 31, 2023 and 2022 on the basis of contractual payments of principal portion which have not been discounted:
At December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
Due within |
|
|
Due between |
|
|
Due beyond |
|
|
Total |
|
||||
|
|
(EUR thousand) |
|
|||||||||||||
Bank overdrafts and short-term loan facilities |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Borrowings from banks (*) |
|
|
|
|
|
|
|
|
— |
|
|
|
|
|||
Notes (*) |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||
Lease liabilities (**) |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Other financial liabilities |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Trade payables |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Tax payables |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Other liabilities (***) |
|
|
|
|
|
|
|
|
— |
|
|
|
|
|||
Employee benefits |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
||||
(*) The corresponding balance reported in the consolidated financial statement position is EUR
(**) The corresponding balance in the consolidated financial statement position is EUR
(***) Other liabilities are mainly related to payables to personnel and social security institutions.
F-78
At December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
Due within |
|
|
Due between |
|
|
Due beyond |
|
|
Total |
|
||||
|
|
(EUR thousand) |
|
|||||||||||||
Bank overdrafts and short-term loan facilities |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Borrowings from banks (*) |
|
|
|
|
|
|
|
|
— |
|
|
|
|
|||
Notes (*) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|||
Lease liabilities (**) |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Other financial liabilities |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Trade payables |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Tax payables |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Other liabilities (***) |
|
|
|
|
|
|
|
|
— |
|
|
|
|
|||
Employee benefits |
|
|
|
|
|
— |
|
|
|
|
|
|
|
|||
Total liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
||||
(*) The corresponding balance reported in the consolidated financial statement position is EUR
(**) The corresponding balance in the consolidated financial statement position is EUR
(***) Other liabilities are mainly related to payables to personnel and social security institutions.
Credit risk
Credit risk is the risk of economic loss arising from the failure to collect a receivable. Credit risk encompasses the direct risk of default and the risk of a deterioration of the creditworthiness of the counterparty. The maximum credit risk to which the Group is theoretically exposed is represented by the carrying amounts of the financial assets stated in the consolidated statement of financial position sheet.
Where customers fail to meet payment deadlines, the Group’s financial position may deteriorate. In addition, socio-political events (or country risks) and the general economic performance of individual countries or geographical regions may assume significance also in relation to this aspect. The trade receivable risk is however mitigated by consolidated commercial relations with high-standing pharmaceutical and biologics companies and Group guidelines created for the selection and evaluation of the client portfolio, which may require, where possible and appropriate, further guarantees from customers.
Trade receivables at December 31, 2023, amounting overall to EUR
Commodity risk
With regard to commodity risk, the Group entered into floating market-price contracts for certain utilities. The Group consumes large amounts of natural gas and electricity for its operating activities. The increased volatility in natural gas and electricity prices over the past 12 months has led to the decision to enter into commodity swap contracts.
These contracts, which commenced in February 2023, are expected to reduce the volatility attributable to price fluctuations of natural gas and electricity. Hedging the price volatility of forecasted natural gas and electricity consumption
F-79
is in accordance with the risk management strategy outlined by the Board of Directors. Hedging contracts are referred to the same index to which the supplying contract is based (i.e. PSV Baseload and PUN Baseload).
At December 31, 2023, the Group held the following contracts:
Commodity |
|
Contract notional (volume) |
|
|
Unit |
|
Carrying amount at December 31, 2023 |
|
|
Line item in the |
|
||
|
|
|
|
|
|
|
(EUR thousand) |
|
|
|
|||
Gas |
|
|
|
|
MWh |
|
|
— |
|
|
Current financial liabilities |
|
|
Energy |
|
|
|
|
MWh |
|
|
( |
) |
|
Current financial liabilities |
|
|
Total |
|
|
|
|
|
|
|
( |
) |
|
|
|
|
39. Events after the reporting period
On January 18, 2024, and on March 5, 2024, Stevanato Group entered into
F-80