

Stevanato Group Q2 2024 Financial Results August 6, 2024 Exhibit 99.1

Q2 2024 Financial Results Safe Harbor Statement Forward-Looking Statements This presentation contains certain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that reflect the current views of Stevanato Group S.p.A. (“we”, “our”, “us”, “Stevanato Group” or the “Company”) and which involve known and unknown risks, uncertainties and assumptions because they relate to events and depend on circumstances that will occur in the future whether or not outside the control of the Company. These forward-looking statements include, or may include words such as “will,” ” believe,” “driving,” “expected,” “expect,” “expansion,” “drive,” “remains,” “still,” “continue,” "rising," "growing," "assumes," "well-positioned," and other similar terminology. Forward-looking statements contained in this presentation include, but are not limited to, statements about: our future financial performance, including our revenue, operating expenses and our ability to maintain profitability and operational and commercial capabilities; our ability to capitalize on opportunities, drive long term organic growth, leverage our product portfolio and build shareholder value; our expectations regarding the development of our industry and the competitive environment in which we operate; the expansion of our plants and our expectations to increase production capacity; the global supply chain and our committed orders; the continued global response to COVID-19 and our role in it; customer demand and customers’ ability to destock higher inventories accumulated during the COVID-19 pandemic; the success of the Company's initiatives to optimize the industrial footprint, harmonize processes and enhance supply chain and logistics strategies; the developments in demographic trends; expansions of vaccinations programs and access to healthcare in developing countries; our geographical and industrial footprint; our goals and capital expenditures projects and our strategies and investment plans. These statements are neither promises nor guarantees but involve known and unknown risks, uncertainties and other important factors and circumstances that may cause Stevanato Group’s actual results, performance or achievements to be materially different from its expectations expressed or implied by the forward-looking statements, including, but not limited to the following: (i) our product offerings are highly complex, and, if our products do not satisfy applicable quality criteria, specifications and performance standards, we could experience lost sales, delayed or reduced market acceptance of our products, increased costs and damage to our reputation; (ii) we must develop new products and enhance existing products, adapt to significant technological and innovative changes and respond to introductions of new products by competitors to remain competitive; (iii) if we fail to maintain and enhance our brand and reputation, our business, results of operations and prospects may be materially and adversely affected; (iv) we are highly dependent on our management and employees. Competition for our employees is intense, and we may not be able to attract and retain the highly skilled employees that we need to support our business and our intended future growth; (v) our business, financial condition and results of operations depend upon maintaining our relationships with suppliers and service providers; (vi) our business, financial condition and results of operations depend upon the availability and price of high-quality materials and energy supply and our ability to contain production costs; (vii) significant interruptions in our operations could harm our business, financial condition and results of operations; (viii) as a consequence of the COVID-19 pandemic, global sales of syringes and vials to and for vaccination programs had increased, resulting in a revenue growth acceleration. The demand for such products may continue to shrink if the need for COVID-19 related solutions continues to decline; (ix) our manufacturing facilities are subject to operating hazards which may lead to production curtailments or shutdowns and have an adverse effect on our business, results of operations, financial condition or cash flows; (x) our business, financial condition and results of operations may be impacted by our ability to successfully expand capacity to meet customer demand; (xi) the loss of a significant number of customers or a reduction in orders from a significant number of customers, including through destocking initiatives or lack of transparency of our products held by customers, could reduce our sales and harm our financial performance; (xii) we may face significant competition in implementing our strategies for revenue growth in light of actions taken by our competitors; (xiii) our global operations are subject to international market risks that may have a material effect on our liquidity, financial condition, results of operations and cash flows; (xiv) we are required to comply with a wide variety of laws and regulations and are subject to regulation by various federal, state and foreign agencies; (xvi given the relevance of our activities in the healthcare sector, investments by non-Italian entities in the Company, as well as certain asset disposals by the Company, may be subject to the prior authorization of the Italian Government (so called “golden powers”); (xvi) if relations between China and the United States deteriorate, our business in the United States and China could be materially and adversely affected; (xvii) cyber security risks and the failure to maintain the confidentiality, integrity and availability of our computer hardware, software and internet applications and related tools and functions, could result in damage to our reputation, data integrity and/or subject us to costs, fines or lawsuits under data privacy or other laws or contractual requirements; (xviii) our trade secrets may be misappropriated or disclosed, and confidentiality agreements with directors, employees and third parties may not adequately prevent disclosure of trade secrets and protect other proprietary information; (ixx) if we are unable to obtain and maintain patent protection for our technology, products and potential products, or if the scope of the patent protection obtained is not sufficiently broad, we may not be able to compete effectively in our markets; (xx) we depend in part on proprietary technology licensed from others. If we lose our existing licenses or are unable to acquire or license additional proprietary rights from third parties, we may not be able to continue developing our potential products; and (xxi) we are obligated to maintain proper and effective internal control over financial reporting. Our internal controls may not be determined to be effective, which may adversely affect investor confidence in us and, as a result, the value of our ordinary shares; and any other risk described under the headings “Risk Factors”, “Operating and Financial Review and Prospects” and “Business” in our most recent Annual Report on Form 20-F filed with the U.S. Securities and Exchange Commission. This list is not exhaustive. We caution you therefore against relying on these forward-looking statements and we qualify all of our forward-looking statements by these cautionary statements. These forward-looking statements speak only as at their dates. The Company undertakes no obligation to update any forward-looking statement or statements to reflect events or circumstances after the date on which such statement is made or to reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible to predict all of these factors. Further, the Company cannot assess the impact of each such factor on our business or the extent to which any factor, or combination of factors, may cause actual results to be materially different from those contained in any forward-looking statements. For a description of certain additional factors that could cause the Company’s future results to differ from those expressed in any such forward-looking statements, refer to the risk factors discussed in our most recent Annual Report on Form 20-F filed with the U.S. Securities and Exchange Commission. Non-GAAP Financial Information This presentation contains non-GAAP financial measures. Please refer to the tables included in this presentation for a reconciliation of non-GAAP financial measures. Management monitors and evaluates its operating and financial performance using several non-GAAP financial measures, including Constant Currency Revenue, EBITDA, Adjusted EBITDA, Adjusted EBITDA Margin, Adjusted Operating Profit, Adjusted Operating Profit Margin, Adjusted Income Taxes, Adjusted Net Profit, Adjusted Diluted EPS, Capital Employed, Net Cash, Free Cash Flow and CAPEX. The Company believes that these non-GAAP financial measures provide useful and relevant information regarding its performance and improve its ability to assess its financial condition. While similar measures are widely used in the industry in which the Company operates, the financial measures it uses may not be comparable to other similarly titled measures used by other companies, nor are they intended to be substitutes for measures of financial performance or financial position as prepared in accordance with IFRS. Accordingly, you should not place undue reliance on any non-IFRS financial measures contained in this presentation.

Q2 2024 Financial Results Stevanato Group Second Quarter 2024 Financial Results Earnings Call Franco Stevanato Executive Chairman & CEO Marco Dal Lago CFO Lisa Miles SVP, IR

Franco Stevanato Executive Chairman & Chief Executive Officer Q2 2024 Financial Results
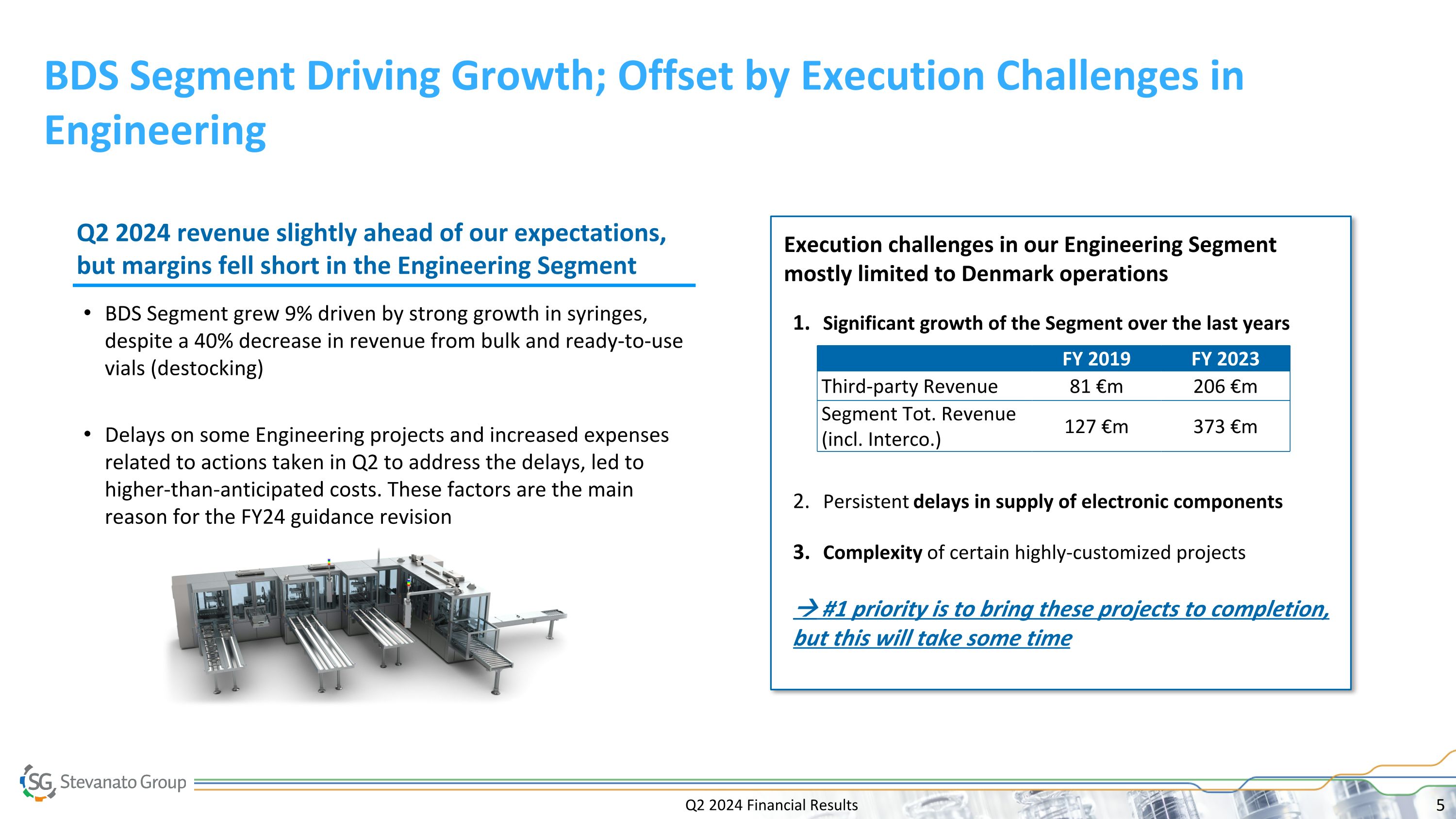
Execution challenges in our Engineering Segment mostly limited to Denmark operations Q2 2024 Financial Results BDS Segment Driving Growth; Offset by Execution Challenges in Engineering Significant growth of the Segment over the last years Persistent delays in supply of electronic components Complexity of certain highly-customized projects #1 priority is to bring these projects to completion, but this will take some time FY 2019 FY 2023 Third-party Revenue 81 €m 206 €m Segment Tot. Revenue (incl. Interco.) 127 €m 373 €m BDS Segment grew 9% driven by strong growth in syringes, despite a 40% decrease in revenue from bulk and ready-to-use vials (destocking) Delays on some Engineering projects and increased expenses related to actions taken in Q2 to address the delays, led to higher-than-anticipated costs. These factors are the main reason for the FY24 guidance revision Q2 2024 revenue slightly ahead of our expectations, but margins fell short in the Engineering Segment
Q2 2024 Financial Results Ongoing Actions to Drive Improvement; Demand Remains Robust Optimize Engineering footprint Harmonize industrial processes among sites Enhance supply chain and logistical strategies As a matter of example: Consolidated activities of two Denmark sites into a single location Implemented cross-site plan to support Denmark teams with our technical specialists from Italy We expect this will also increase standardization of our technologies and processes across the Segment While costs of these initiatives will impact our FY 2024, they will help us optimize our operational structure, maximize efficiencies, and secure the success of ongoing projects Demand for Engineering remains favorable, as customers continue to secure manufacturing capacity for fast-growing biologics, and upgrade infrastructure to better align with regulatory requirements Ongoing initiatives to improve efficiencies across the Eng. Segment Also, we are still navigating through market-wide vial destocking. Positive signals with orders from smaller markets (e.g., Latin America) In larger markets, we still expect a more gradual recovery. Customers are sharing forecasts that point to improving demand and are beginning production planning. We are cautiously optimistic that vial orders will pick up at the tail-end of 2024, starting with bulk vials

Q2 2024 Financial Results Demand-Driven Capacity Expansion Update Latina, Italy Hosted 40 customers for the new plant opening; Two plants at Latina Campus. Ongoing PFS capacity expansion; multi-year ramp up Expansion of EZ-fill® cartridge capacity for anchor customer transitioning to RTU (activities launch in 2025). Expansion includes the installation of new lines in the new plant, and the upgrade of already-operational lines in the original Latina plant. Harmonization is part of our wider strategy to enhance efficiency and production capacity, reduce lead times, and expand our offer of premium solutions. Fishers (IN), U.S. Validation activities progressing as planned; progressing through first set of customer audits Took delivery of 2nd and 3rd high-performance syringe lines; installation and qualification underway On track to launch commercial production and generate revenue in Q3 24 China With our focus on the execution in Latina and Fishers, we decided to further postpone potential expansion in China for the coming 12 months. We believe we have sufficient capacity to support the demand in the region.
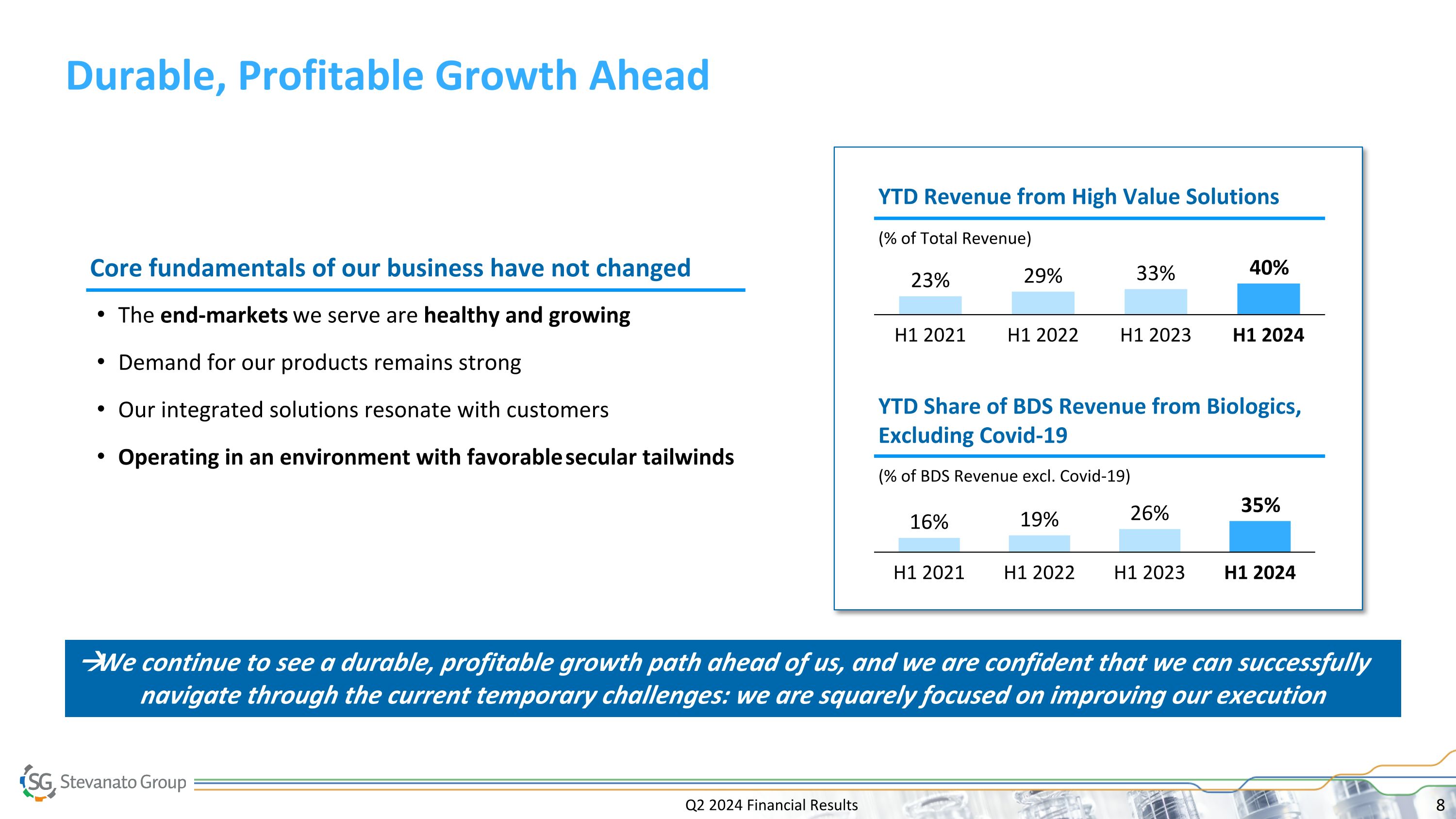
Q2 2024 Financial Results Durable, Profitable Growth Ahead The end-markets we serve are healthy and growing Demand for our products remains strong Our integrated solutions resonate with customers Operating in an environment with favorable secular tailwinds H1 2021 H1 2022 H1 2023 H1 2024 (% of BDS Revenue excl. Covid-19) YTD Share of BDS Revenue from Biologics, Excluding Covid-19 H1 2021 H1 2022 H1 2023 H1 2024 (% of Total Revenue) YTD Revenue from High Value Solutions Core fundamentals of our business have not changed We continue to see a durable, profitable growth path ahead of us, and we are confident that we can successfully navigate through the current temporary challenges: we are squarely focused on improving our execution
Marco Dal Lago Chief Financial Officer Q2 2024 Financial Results
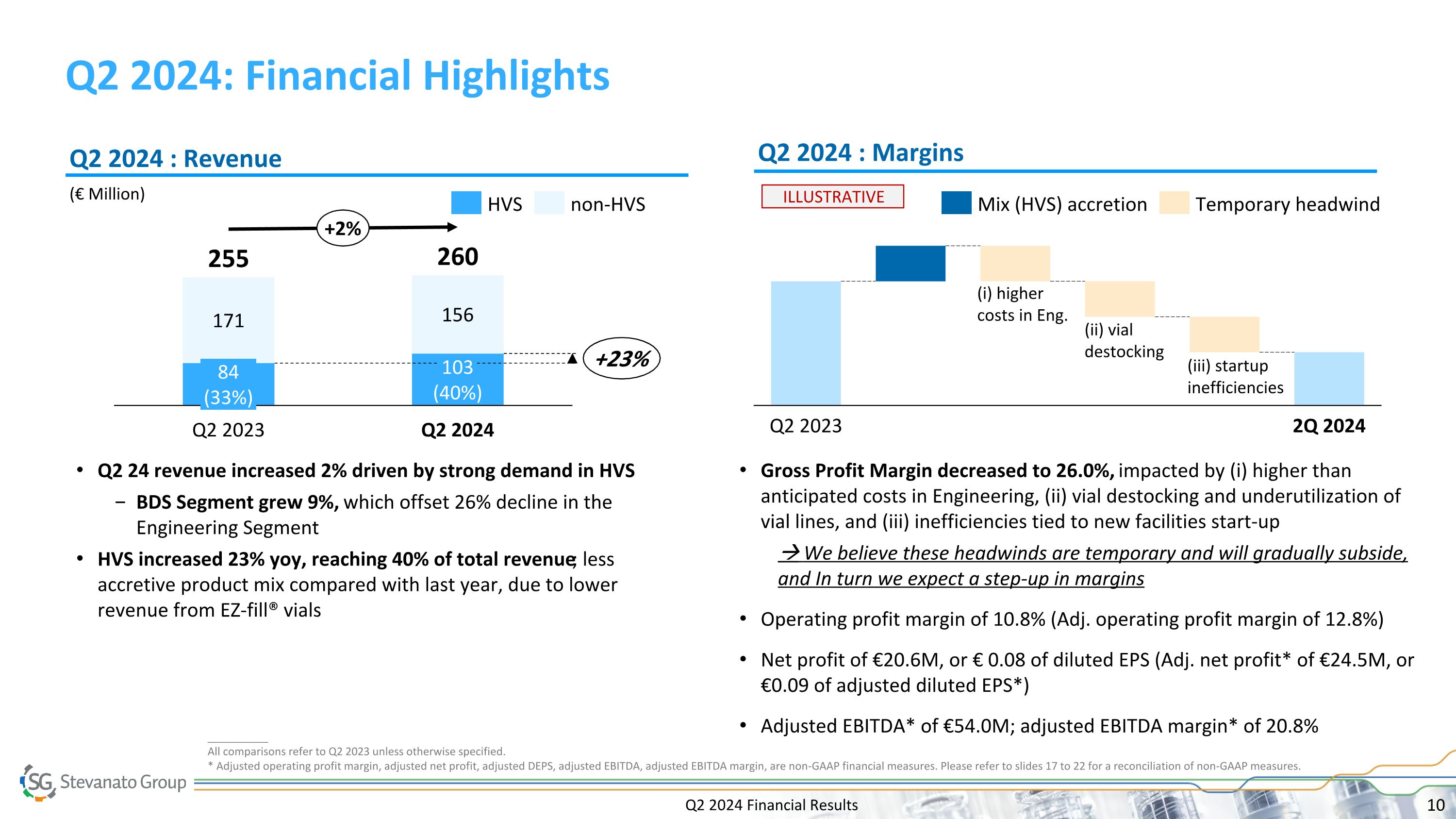
Q2 2024 Financial Results Q2 2024: Financial Highlights __________ All comparisons refer to Q2 2023 unless otherwise specified. * Adjusted operating profit margin, adjusted net profit, adjusted DEPS, adjusted EBITDA, adjusted EBITDA margin, are non-GAAP financial measures. Please refer to slides 17 to 22 for a reconciliation of non-GAAP measures. (€ Million) Q2 2024 : Revenue Q2 2024 : Margins Base business 171 84 (33%) Q2 2023 156 103 (40%) Q2 2024 255 260 +23% +2% HVS non-HVS Q2 2023 2Q 2024 ILLUSTRATIVE (ii) vial destocking (iii) startup inefficiencies Q2 24 revenue increased 2% driven by strong demand in HVS BDS Segment grew 9%, which offset 26% decline in the Engineering Segment HVS increased 23% yoy, reaching 40% of total revenue; less accretive product mix compared with last year, due to lower revenue from EZ-fill® vials Gross Profit Margin decreased to 26.0%, impacted by (i) higher than anticipated costs in Engineering, (ii) vial destocking and underutilization of vial lines, and (iii) inefficiencies tied to new facilities start-up We believe these headwinds are temporary and will gradually subside, and In turn we expect a step-up in margins Operating profit margin of 10.8% (Adj. operating profit margin of 12.8%) Net profit of €20.6M, or € 0.08 of diluted EPS (Adj. net profit* of €24.5M, or €0.09 of adjusted diluted EPS*) Adjusted EBITDA* of €54.0M; adjusted EBITDA margin* of 20.8% Mix (HVS) accretion Temporary headwind (i) higher costs in Eng.
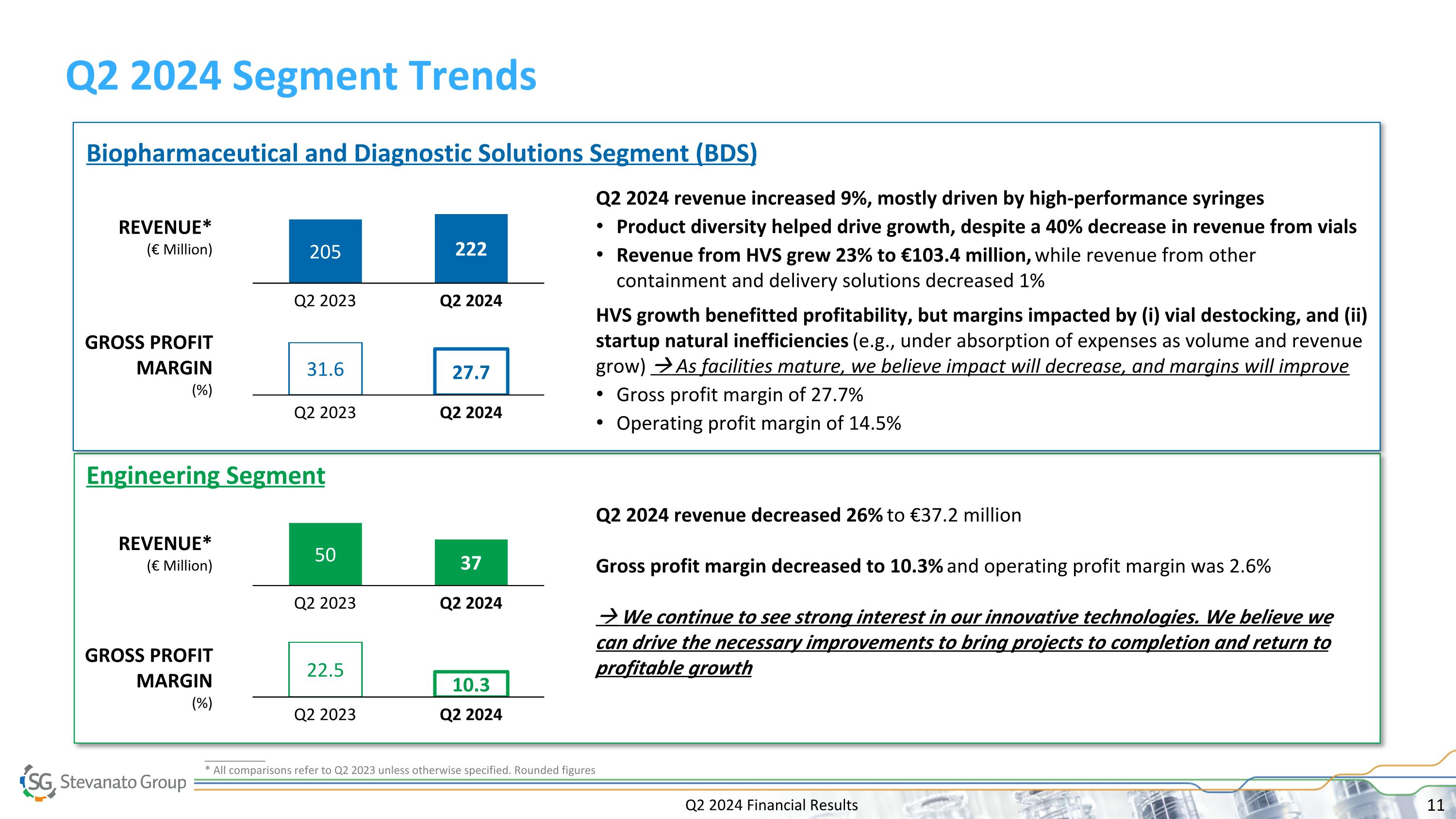
Q2 2024 Financial Results Q2 2024 Segment Trends 205 Q2 2023 222 Q2 2024 Q2 2024 revenue increased 9%, mostly driven by high-performance syringes Product diversity helped drive growth, despite a 40% decrease in revenue from vials Revenue from HVS grew 23% to €103.4 million, while revenue from other containment and delivery solutions decreased 1% HVS growth benefitted profitability, but margins impacted by (i) vial destocking, and (ii) startup natural inefficiencies (e.g., under absorption of expenses as volume and revenue grow) As facilities mature, we believe impact will decrease, and margins will improve Gross profit margin of 27.7% Operating profit margin of 14.5% Biopharmaceutical and Diagnostic Solutions Segment (BDS) REVENUE* (€ Million) GROSS PROFIT MARGIN (%) Engineering Segment 31.6 Q2 2023 27.7 Q2 2024 50 Q2 2023 37 Q2 2024 Q2 2024 revenue decreased 26% to €37.2 million Gross profit margin decreased to 10.3% and operating profit margin was 2.6% We continue to see strong interest in our innovative technologies. We believe we can drive the necessary improvements to bring projects to completion and return to profitable growth REVENUE* (€ Million) GROSS PROFIT MARGIN (%) 22.5 Q2 2023 10.3 Q2 2024 __________ * All comparisons refer to Q2 2023 unless otherwise specified. Rounded figures
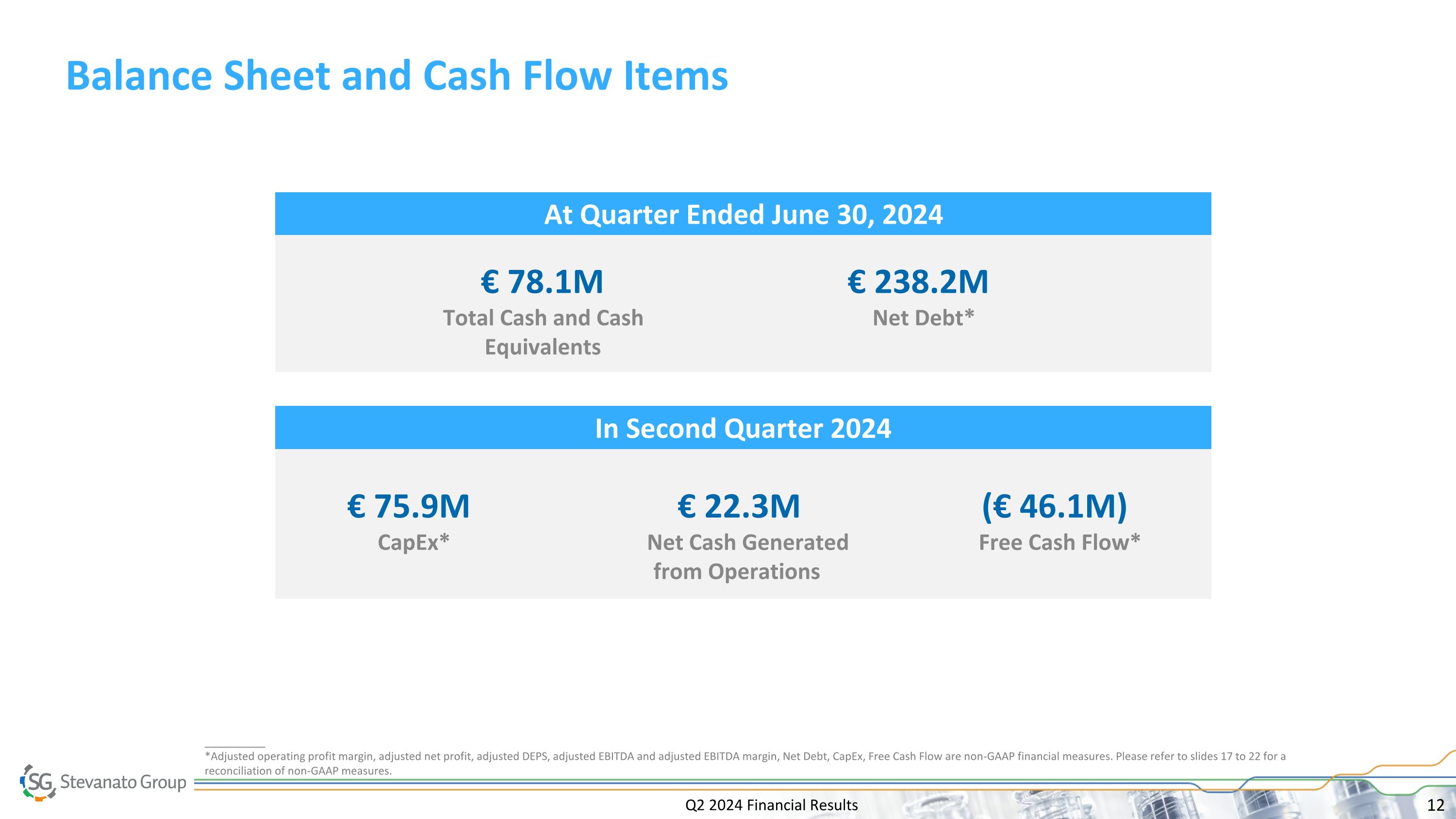
Q2 2024 Financial Results Balance Sheet and Cash Flow Items In Second Quarter 2024 At Quarter Ended June 30, 2024 (€ 46.1M) Free Cash Flow* € 238.2M Net Debt* € 78.1M Total Cash and Cash Equivalents € 75.9M CapEx* € 22.3M Net Cash Generated from Operations __________ *Adjusted operating profit margin, adjusted net profit, adjusted DEPS, adjusted EBITDA and adjusted EBITDA margin, Net Debt, CapEx, Free Cash Flow are non-GAAP financial measures. Please refer to slides 17 to 22 for a reconciliation of non-GAAP measures.
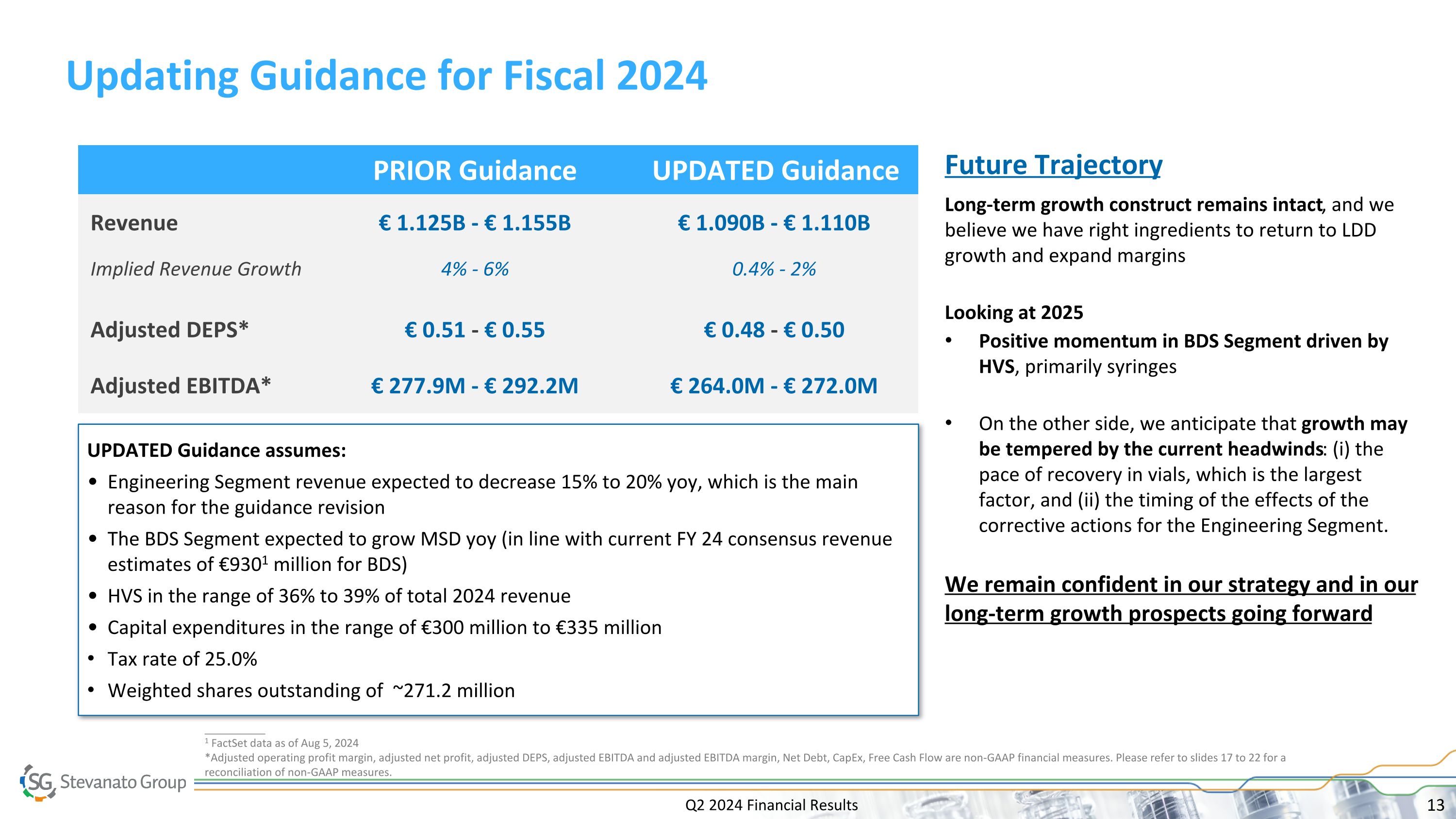
Q2 2024 Financial Results Updating Guidance for Fiscal 2024 UPDATED Guidance assumes: Engineering Segment revenue expected to decrease 15% to 20% yoy, which is the main reason for the guidance revision The BDS Segment expected to grow MSD yoy (in line with current FY 24 consensus revenue estimates of €9301 million for BDS) HVS in the range of 36% to 39% of total 2024 revenue Capital expenditures in the range of €300 million to €335 million Tax rate of 25.0% Weighted shares outstanding of ~271.2 million PRIOR Guidance UPDATED Guidance Revenue € 1.125B - € 1.155B € 1.090B - € 1.110B Implied Revenue Growth 4% - 6% 0.4% - 2% Adjusted DEPS* € 0.51 - € 0.55 € 0.48 - € 0.50 Adjusted EBITDA* € 277.9M - € 292.2M € 264.0M - € 272.0M __________ 1 FactSet data as of Aug 5, 2024 *Adjusted operating profit margin, adjusted net profit, adjusted DEPS, adjusted EBITDA and adjusted EBITDA margin, Net Debt, CapEx, Free Cash Flow are non-GAAP financial measures. Please refer to slides 17 to 22 for a reconciliation of non-GAAP measures. Future Trajectory Long-term growth construct remains intact, and we believe we have right ingredients to return to LDD growth and expand margins Looking at 2025 Positive momentum in BDS Segment driven by HVS, primarily syringes On the other side, we anticipate that growth may be tempered by the current headwinds: (i) the pace of recovery in vials, which is the largest factor, and (ii) the timing of the effects of the corrective actions for the Engineering Segment. We remain confident in our strategy and in our long-term growth prospects going forward

Franco Stevanato Executive Chairman & Chief Executive Officer Q2 2024 Financial Results
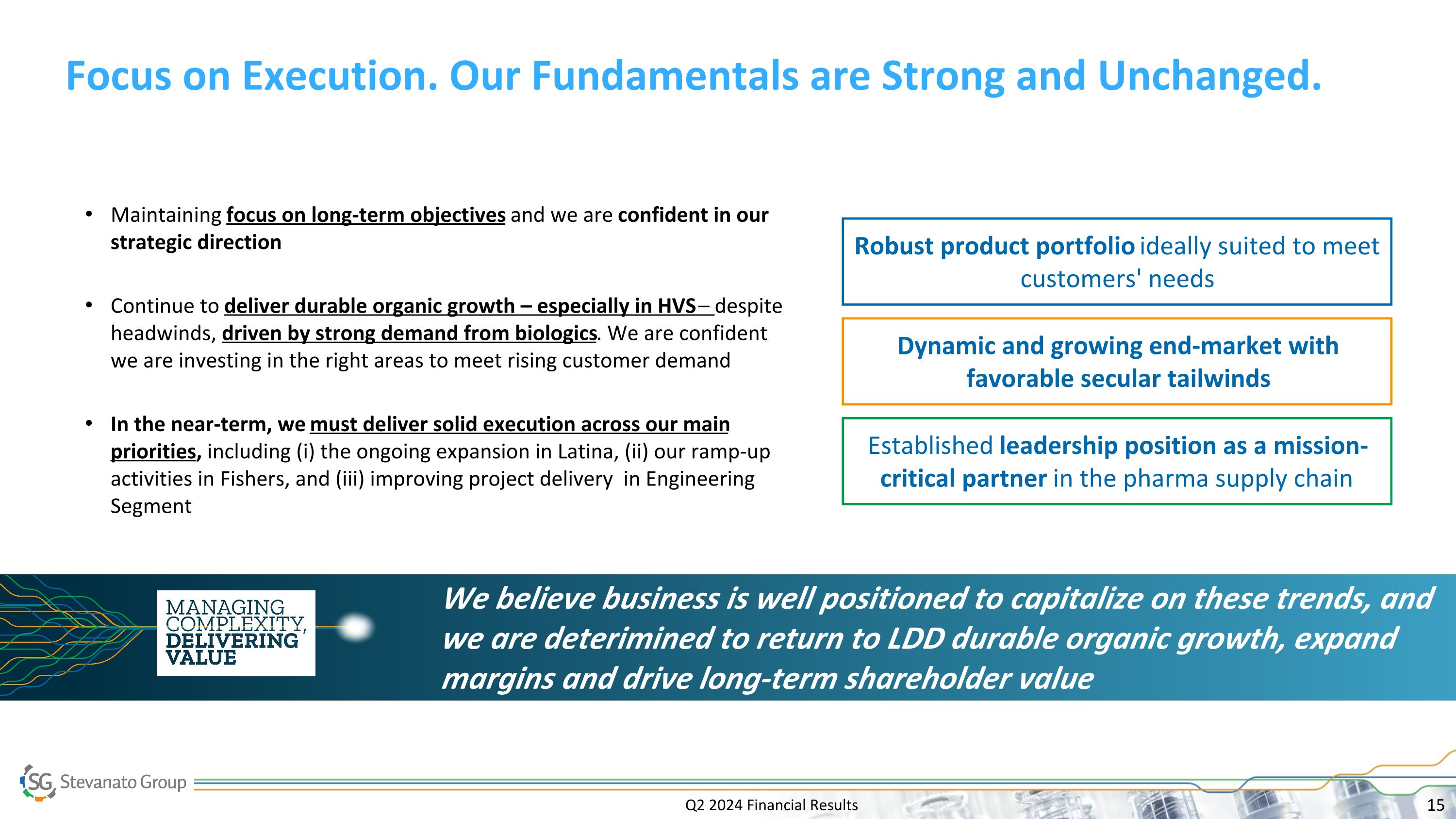
Q2 2024 Financial Results Focus on Execution. Our Fundamentals are Strong and Unchanged. Maintaining focus on long-term objectives and we are confident in our strategic direction Continue to deliver durable organic growth – especially in HVS – despite headwinds, driven by strong demand from biologics. We are confident we are investing in the right areas to meet rising customer demand In the near-term, we must deliver solid execution across our main priorities, including (i) the ongoing expansion in Latina, (ii) our ramp-up activities in Fishers, and (iii) improving project delivery in Engineering Segment Robust product portfolio ideally suited to meet customers' needs Dynamic and growing end-market with favorable secular tailwinds Established leadership position as a mission-critical partner in the pharma supply chain We believe business is well positioned to capitalize on these trends, and we are deterimined to return to LDD durable organic growth, expand margins and drive long-term shareholder value

Stevanato Group Q2 2024 Financial Results
This presentation contains non-GAAP financial measures. Please refer to the tables included in this presentation for a reconciliation of non-GAAP measures. Management monitors and evaluates our operating and financial performance using several non-GAAP financial measures, including Constant Currency Revenue, EBITDA, Adjusted EBITDA, Adjusted EBITDA Margin, Adjusted Operating Profit, Adjusted Operating Profit Margin, Adjusted Income Taxes, Adjusted Net Profit, Adjusted Diluted EPS, Capital Employed, Net Cash, Free Cash Flow, and CapEx. We believe that these non-GAAP financial measures provide useful and relevant information regarding our performance and improve our ability to assess our financial condition. While similar measures are widely used in the industry in which we operate, the financial measures we use may not be comparable to other similarly titled measures used by other companies, nor are they intended to be substitutes for measures of financial performance or financial position as prepared in accordance with IFRS. Reconciliation of Non-GAAP Financial Measures Q2 2024 Financial Results
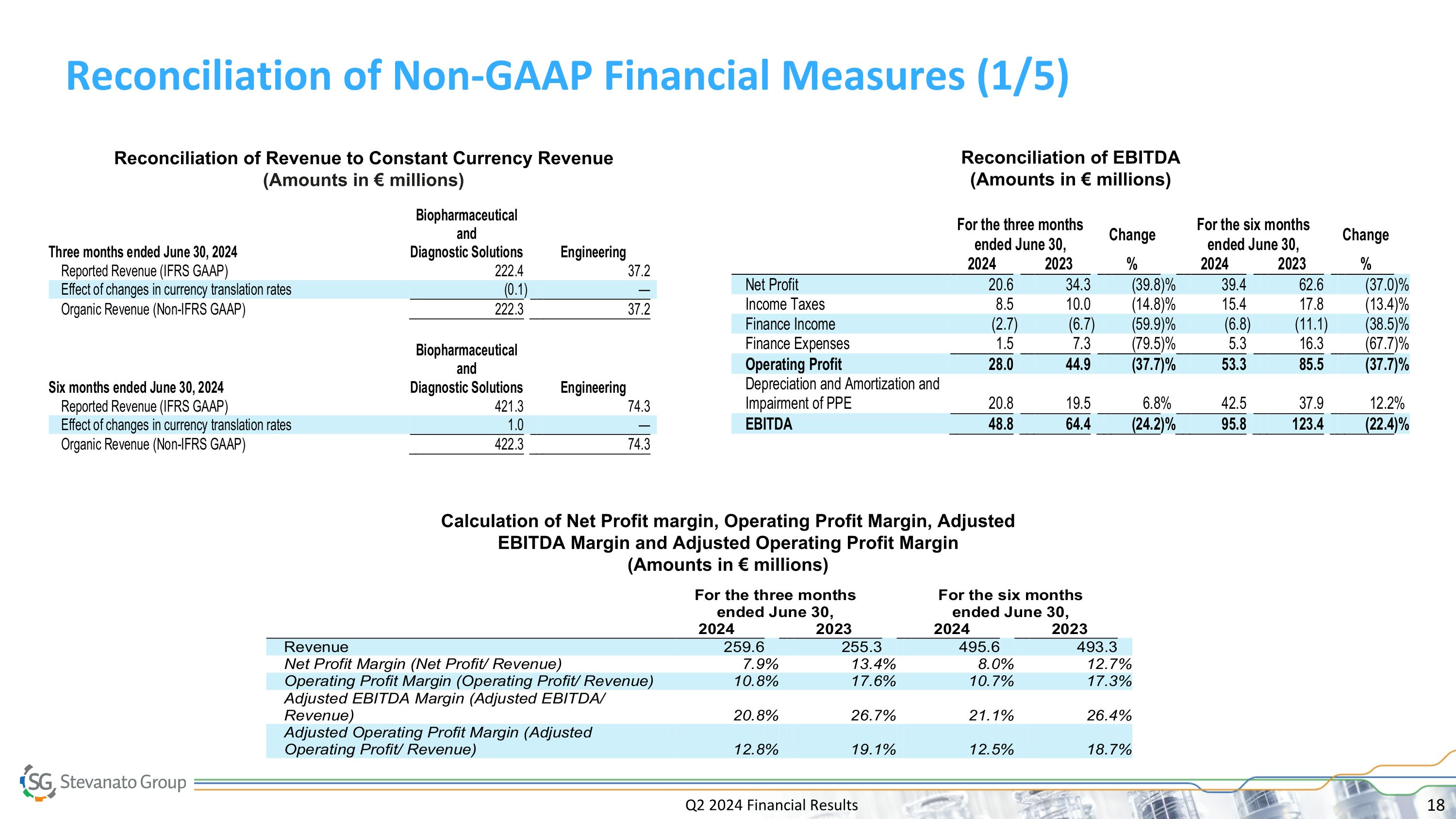
Q2 2024 Financial Results Reconciliation of Non-GAAP Financial Measures (1/5) Reconciliation of EBITDA (Amounts in € millions) Calculation of Net Profit margin, Operating Profit Margin, Adjusted EBITDA Margin and Adjusted Operating Profit Margin (Amounts in € millions) Reconciliation of Revenue to Constant Currency Revenue (Amounts in € millions)
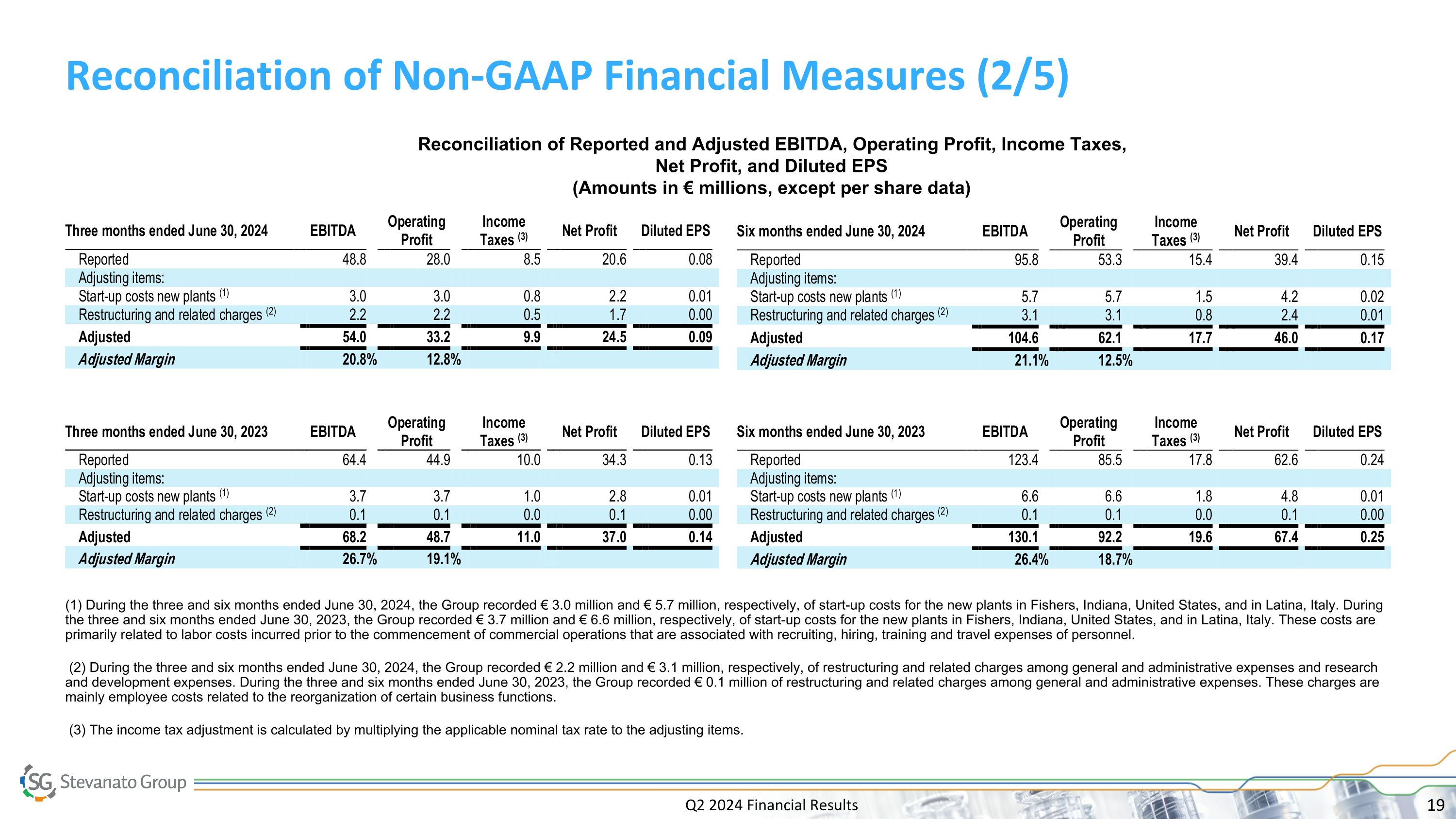
Q2 2024 Financial Results Reconciliation of Non-GAAP Financial Measures (2/5) Reconciliation of Reported and Adjusted EBITDA, Operating Profit, Income Taxes, Net Profit, and Diluted EPS (Amounts in € millions, except per share data) (1) During the three and six months ended June 30, 2024, the Group recorded € 3.0 million and € 5.7 million, respectively, of start-up costs for the new plants in Fishers, Indiana, United States, and in Latina, Italy. During the three and six months ended June 30, 2023, the Group recorded € 3.7 million and € 6.6 million, respectively, of start-up costs for the new plants in Fishers, Indiana, United States, and in Latina, Italy. These costs are primarily related to labor costs incurred prior to the commencement of commercial operations that are associated with recruiting, hiring, training and travel expenses of personnel. (2) During the three and six months ended June 30, 2024, the Group recorded € 2.2 million and € 3.1 million, respectively, of restructuring and related charges among general and administrative expenses and research and development expenses. During the three and six months ended June 30, 2023, the Group recorded € 0.1 million of restructuring and related charges among general and administrative expenses. These charges are mainly employee costs related to the reorganization of certain business functions. (3) The income tax adjustment is calculated by multiplying the applicable nominal tax rate to the adjusting items.
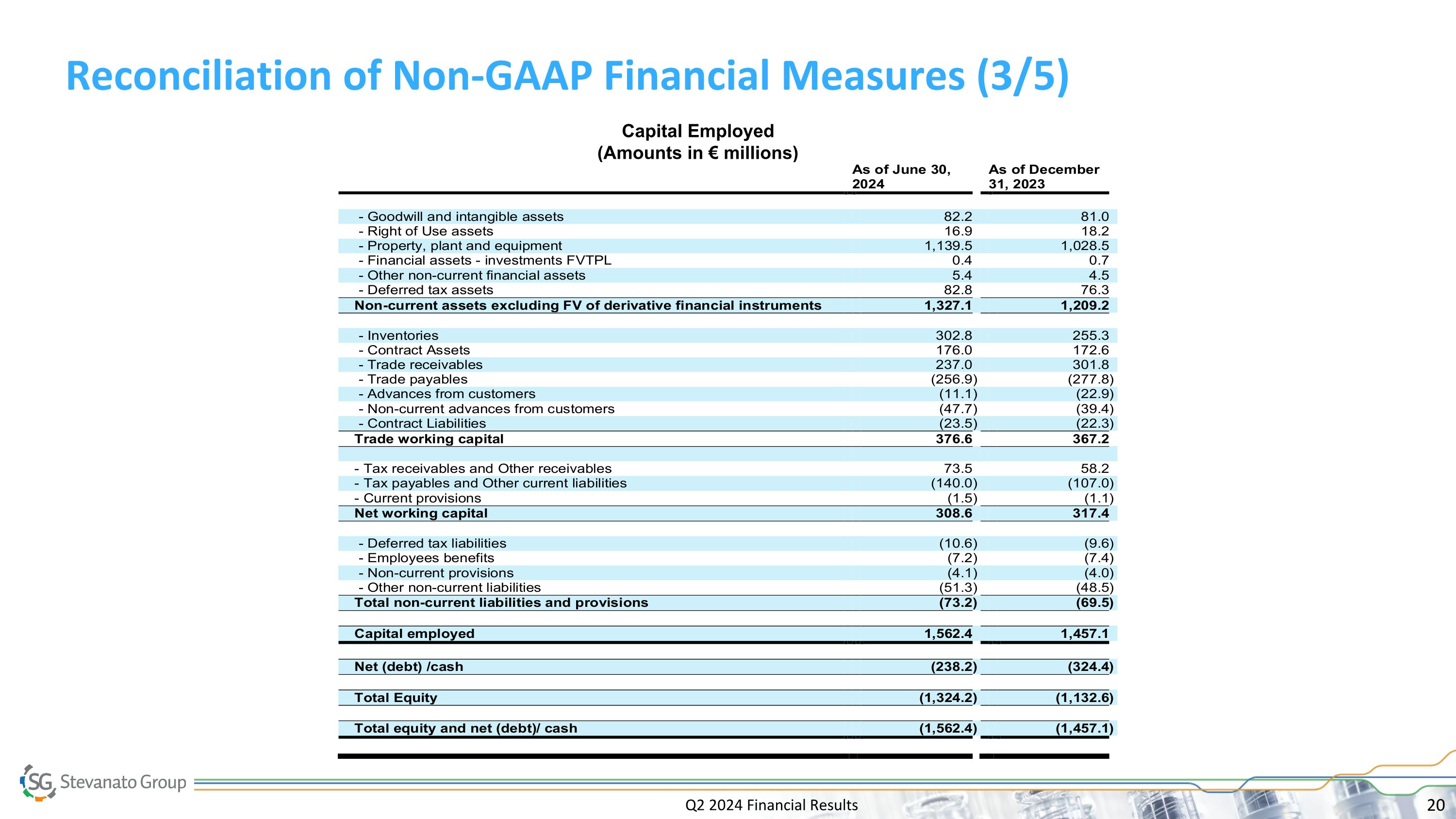
Q2 2024 Financial Results Reconciliation of Non-GAAP Financial Measures (3/5) Capital Employed (Amounts in € millions)
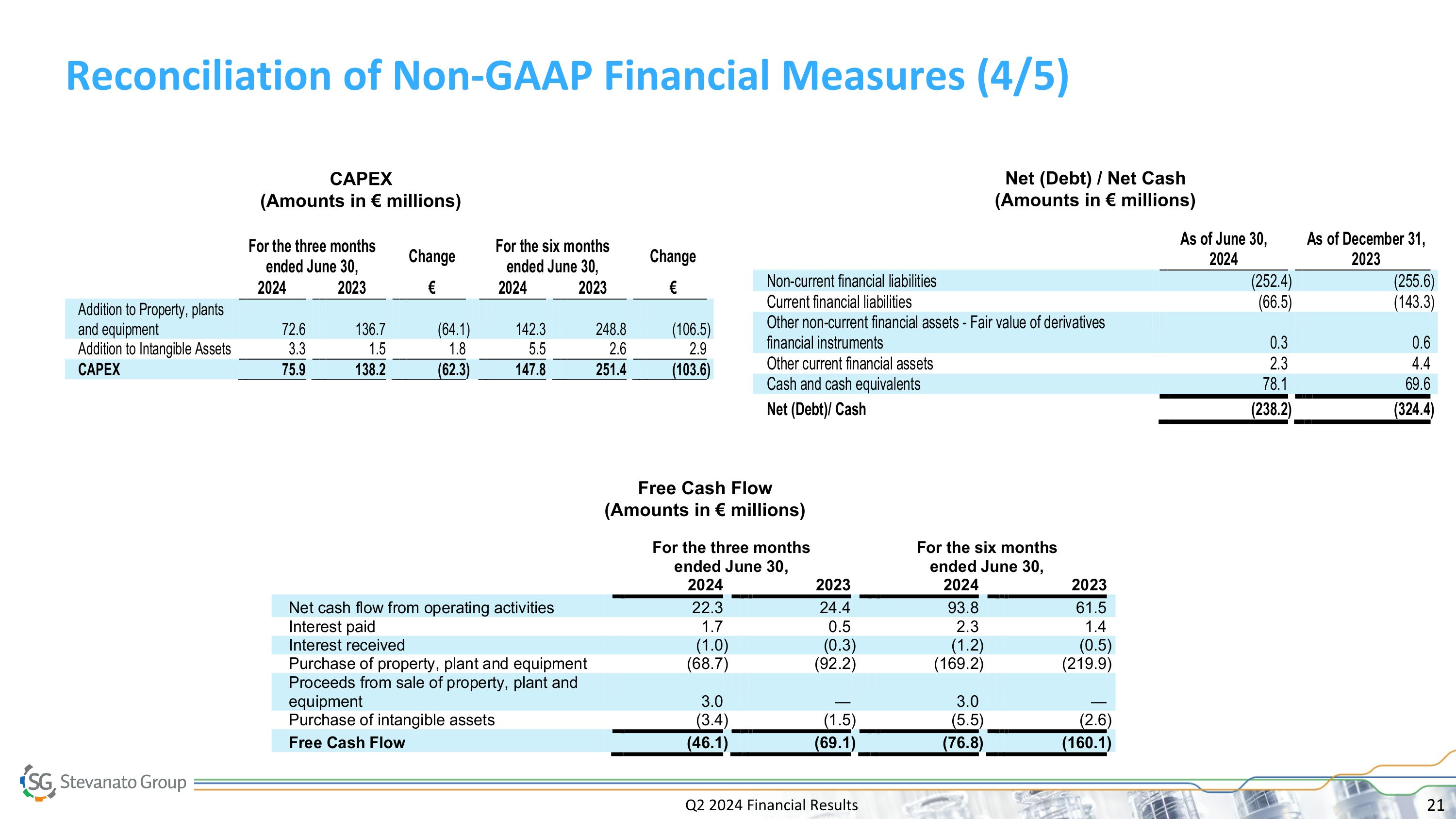
Q2 2024 Financial Results Reconciliation of Non-GAAP Financial Measures (4/5) Net (Debt) / Net Cash (Amounts in € millions) Free Cash Flow (Amounts in € millions) CAPEX (Amounts in € millions)
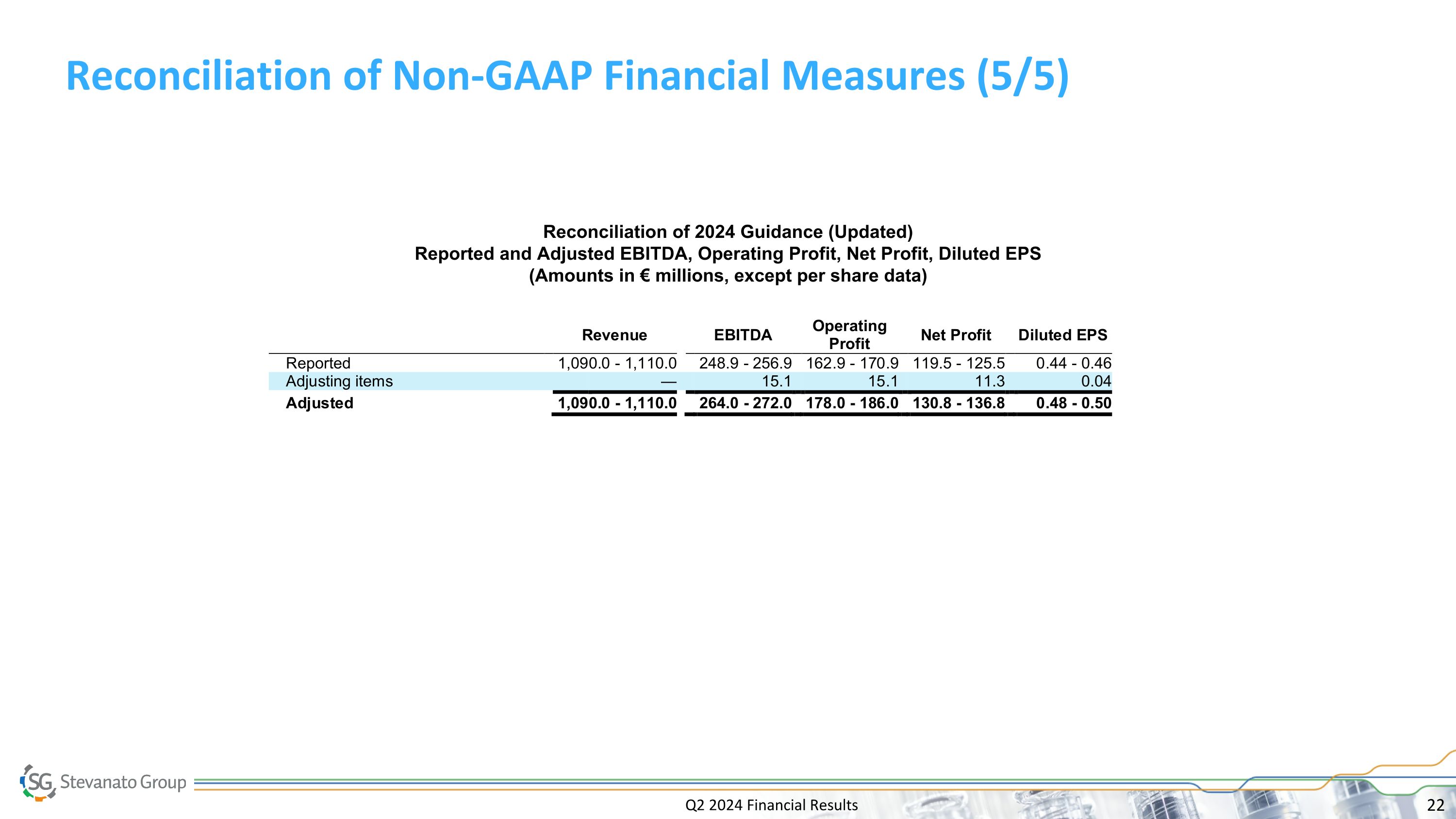
Q2 2024 Financial Results Reconciliation of Non-GAAP Financial Measures (5/5) Reconciliation of 2024 Guidance (Updated) Reported and Adjusted EBITDA, Operating Profit, Net Profit, Diluted EPS (Amounts in € millions, except per share data)