Exhibit 99.2

Sustainability Report 2021 Stevanato Group
Exhibit 99.2

Sustainability Report 2021 Stevanato Group

Table of contents Letter to Stakeholders IV Methodological note VII STEVANATO GROUP 9 1.1 Highlights 10 1.2 Story and evolution 12 1.3 Mission, Vision and Values 24 1.4 The company structure and main corporate functions 27 1.5 Ethics, integrity and compliance 34 STEVANATO GROUP AND SUSTAINABILITY 37 2.1 Approach to sustainability 38 2.2 Certifications and awards 45 2.3 Participation in organizations and associations 49 ECONOMIC SUSTAINABILITY 52 3.1 Stakeholder value creation 53 3.2 Key financial results 55 RESEARCH, DEVELOPMENT, INNOVATION AND PRODUCT RESPONSIBILITY 60 4.1 Stevanato Group products, technologies and services 61 4.1.1 Biopharmaceutical and Diagnostic solutions 64 4.1.2 Engineering 70 1 2 3 4

Sustainability Report—2021 4.2 R&D and Innovation 71 4.2.1 R&D for Drug containment solutions (DCS) 72 4.2.2 R&D on Drug Delivery Systems (DDS) 73 4.2.3 R&D on Engineering 74 4.2.4 Analytical Services 78 4.3 Product quality and responsibility 80 ATTENTION TO HUMAN CAPITAL 83 5.1 Stevanato Group’s human resources5 84 5.2 Employee management and development 92 5.3 Occupational Health & Safety 100 SUPPLY CHAIN RESPONSIBILITY 108 6.1 Responsible supply chain & procurement 109 ATTENTION TO THE ENVIRONMENT 117 7.1 Stevanato Group’s commitment to the environment 118 7.2 Energy Consumption and GHG Emissions 120 7.3 Water management 124 7.4 Waste management 129 THE LINK WITH THE TERRITORY 133 8.1 Local communities engagement 134 Topic Boundaries 138 GRI Content Index 139 Independent Audit Report 144 5 6 7 8

Sustainability Report—2021 Letter to Stakeholders Giovanni Stevanato had a dream and a vision. He wanted to create world-class glass manufacturing activities in Piom-bino Dese, Italy, and set a standard of excellence in the industry. Seventy years ago, we took over his legacy, proudly building a global manufacturing enterprise with an international team of dedicated professionals driven by his same passion and desire to keep learning and improving. In 2021, we joined the New York Stock Exchange and began the next chapter in our storied history. The listing will further strengthen our integrated offerings, increase Stevanato’s market penetration across our business segments and accelerate our growth. We are proud of what we have accomplished and excited for our future as a listed company. As a global operation with 16 sites around the world, we foster a culture that values diversity and respects individual contributions that spark innovation, leadership and exceptional performance. Our strength comes from working together as One Team towards a common goal: enhance the integrity of medicines to help people live a better life. To do so, we work alongside customers, partners and the scientific community to find effective, high-quality solutions to new problems, pushing the limits of knowledge further and further while always keeping the customer and patient at the center of what we do. Along the way, our Values and Guiding Principles provide a framework for our ethical-social responsibilities and ensure our accountability. As with any organization, we have been through some challenging times. There were moments when we modified our plans and changed direction in order to meet the needs of our stakeholders. In those instances, we evaluated, adjusted and altered our approach, reacting to, or sometimes even anticipating, market demands. But we held steadfastly to our belief that the best way to make a difference is by transforming an obstacle into an opportunity for growth. We are now a leading global provider of drug containment, delivery and diagnostic solutions to the biopharma-ceutical and life sciences industries. We deliver an integrated, end-to-end portfolio of products, processes and services that address customer needs across the entire drug life cycle at each of the development, clinical and commercial stages. Our core capabilities in scientific research and development, technical innovation and engineering excellence are central to our ability to offer integrated, value-added solutions to our clients. Through our investments in research and development and with our global reach, we have secured a leadership position in the drug development and delivery value chain. Over our 70-year history, we have earned a leading reputation for high quality and reliability, enabling us to become the partner of choice for more than 700 companies globally, including 41 of the top 50 pharmaceutical companies (which comprise all of the top 15), and eight of the top ten in-vitro diagnostic companies, as measured by 2020 revenue data collected by Global Data. We also serve 15 of the top 20 biotechnology companies by market capitalization in the NASDAQ Biotechnology Index and over 100 biotechnology customers in total. IV |

Sustainability Report—2021 Our priority is to provide flexible, integrated, end-to-end solutions that preserve the integrity of pharmaceutical products, enabling our customers to deliver safe and effective drug products to patients quickly and efficiently while reducing the time to market, total cost of ownership (i.e., logistic, storage and personnel costs) and supply chain risk. We achieve this by developing our products in partnership with our customers and leveraging our scientific research, technical expertise, engineering and manufacturing excellence in order to meet the exact specifications and quality standards required for their drug products. In 2021, we significantly ramped up our efforts to align Stevanato’s corporate culture with relevant environmental, social and governance elements. We are committed to embedding sustainability values into policies and practices and establishing a culture of integrity. We are also dedicated to satisfying our sta- keholders’ interests and expectations. In this respect, we strive to grow and support our customers while making a positive impact for the benefit of all, in everything we do. We consider ourselves an interdependent and responsible member of the community we live in and, as such, we do our part to promote initiatives and solutions that foster the health and wellbeing of society and the planet. For the first time, we have voluntarily elected to provide complete, transparent non-financial reporting to our stakeholders and investors. Our 2021 Sustainability Report not only considers the Company’s financial and economic results, but also highlights the environmental, social and governance (ESG) performance of the Group. This is our first published Sustainability Report subject to limited assurance and prepared in accordance with the Global Reporting Initiative (GRI) Standards, which demonstrates our unwavering commitment to sustainability. V |

Sustainability Report—2021 In regards to ESG governance, the Board has shown its strong commitment through the constitution of the ESG Committee. Several functions are involved in the management of ESG matters according to each relevant area of accountability. As a baseline, Stevanato Group tackles ESG risks and opportunities in accordance with current legislation and regulations. In the last quarter of 2021, real GDP growth in the euro area slowed down after two quarters of strong expansion but reached its pre-pandemic level at the end of 2021. Hence, GDP is estimated to have increased by 5.2% in 2021 following a 6.4% decline in 2020. The U.S. economy recovered from the recession caused by Covid-19 with GDP returning to pre-pandemic levels as early as mid-2021. Expansionary growth in China, however, remained weak. In the fourth quarter of 2021, China’s GDP growth rose to 1.6% over the previous quarter, an increase that brought twelve-month growth to 8.1% in 2021. The emergence of the Omicron variant continues to pose risks to growth in the short term. Thus, growth is likely to remain subdued in the first quarter of 2022 as the ongoing pandemic continues to affect economic activity. High uncertainty also stems from the conflict between Russia and Ukraine which erupted at the end of February 2022, posing potentially severe economic consequences. Since the outbreak of COVID-19, we have increased production capacity to support our customers’ efforts to provide a rapid response. In this context, we have been providing: (i) glass vials and syringes to approximately 90% of the currently marketed vaccine programs, according to estimates based on public information (WHO, EMA, FDA); and (ii) plastic diagnostic consumables for the detection and diagnosis of COVID19. COVID-19 has generated increased demand for our products and services, further enabling us to accelerate our growth strategy. Going forward, we expect demand for syringes, vials and related products and services to remain elevated as the COVID-19 vaccine and treatment programs continue to roll-out globally, and as our customers contemplate the transition from mul-ti-dose to single-dose formats. In addition, we expect continued tailwinds as epidemic preparedness, including the ongoing global COVID-19 vaccine rollout, booster shot distribution and new vaccination programs, remains a priority for governments. The Company has made meaningful progress and significant milestones were achieved in 2021. None of this would have been possible without the invaluable contribution of our dedicated and passionate employees. We would also like to express our sincere appreciation to Stevanato Group’s stakeholders—our suppliers, customers, universities and research centers—as well as our shareholders and Board of Directors, communities, local authority and regulators. You have been, and will continue to be, an integral part of our success. Much has been done, but even more is left to do. Challenges are part of our history and the stepping stones to the future we intend to build, day after day. Franco Stevanato Executive Chairman Stevanato Group S.p.A. Via Molinella 17, 35017 Piombino Dese · Padova · Italy Franco Moro Chief Executive Officer & Chief Operating Officer Stevanato Group S.p.A. Via Molinella 17, 35017 Piombino Dese · Padova · Italy VI |

Sustainability Report—2021 Methodological note Our first official and published Sustainability Report is a vehicle for describing, in a transparent and structured manner, Stevanato Group’s environmental, social and economic achievements in the 2021 financial year (January 1-December 31) and shows the commitment and initiatives undertaken towards the goal of sustainable development. Therefore, the purpose of the information contained in this document is to provide internal and external stakeholders with a representation of Stevanato Group’s business performance, results and impact in relation to the main sustainability topics relating to the 2021 financial year. This document represents the Sustainability Report of the companies belonging to Stevanato Group and its subsidiaries (hereinafter also referred to as the “Company”, “Stevanato”, the “Stevanato Group”, the “Group” or “SG”). The scope of the economic data is the same as the 2021 Consolidated Finan- cial Statement of Stevanato Group. The scope of social and environmental data comprises the companies belonging to Stevanato Group as of December 31, 2021, consolidated on a line-by-line basis within the Group Consolidated Financial Statement. Note that some Group companies, which are not productive or operational, have not been included in the EHS aspect due to limitation of scope (see relevant footnotes). This Report, which is going to be published annually, was prepared mainly with reference to the “Global Reporting Initiative (GRI) Sustainability Reporting Standards”. For the specific GRI Standards 303 (Water and water discharges) and GRI 403 (Occupational health and safety), the most recent 2018 versions have been adopted, while for the specific GRI Standard 306 (Waste), the most recent 2020 version has been used. VII |

Sustainability Report—2021 The GRI Standards used in this report create a common language between the organization and its internal and external stakeholders so that the environmental, social and economic impacts can be communicated and understood. The following GRI disclosures have been referenced within this document using a “GRI-Referenced” approach: 102-1, 102-2, 102-3, 102-4, 102-5, 102-6, 102-7, 102-8, 102-9, 102-10, 102-11, 102-12, 102-13, 102-14, 102-16, 102-18, 102-40, 102-41, 102-42, 102-43, 102-44, 102-45, 102-46, 102-47, 102-48, 102-49, 102-50, 102-51, 102-52, 102-53, 102-54, 102-55, 102-56, 103- 1, 103-2, 103-3, 201-1, 204-1, 205-3, 206-1, 302-1, 303-1, 303-2, 303-3, 303-4, 305-1, 305-2, 306-1, 306-2, 306-3, 307-1, 401-1, 401-2, 403-1, 403-2, 403-3, 403-4, 403-5, 403-6, 403-7, 403-9, 405-1, 406-1, 413-2, 416-2. For more details, please see the table “GRI Content Index” in the appendix. The contents of the Report were selected based on the results of a materiality analysis carried out in 2020, which identified the material topics for the Group and its stakeholders published within this document as described in Chapter 2. The materiality analysis included the identification of possible ESG (Environmental, Social and Governance) topics relevant to the Group, the prioritization of the topics identified, and their approval by SG’s management. Note that the results of the materiality analysis process were examined and approved by the Board of Director at the meeting held on February 18, 2021. In line with the reporting guidelines, the sustainability information presentation reflects the principle of materiality. In regards to the reporting process, a global working group of approximately one hundred individuals was established from across the Group. The working group was coordinated and supported in its endeavors to determine the most significant information per material topic, select the appropriate indicators, collect and validate the narrative, and define the management approach and pertinent data. Specifically, the information included herein was taken both from the Group’s IT system and from the sustainability reporting package (data collection sheets and narrative collection forms). Plant managers across the Group’s local entities were also asked to identify the most important locally-based projects and initiatives, which were described as required. In order to properly manage the reporting process, a Sustainability Reporting Procedure was set up. It defined and illustrated how to prepare the Group’s Sustainability Report in accordance with the Sustainability Reporting Guidelines issued by the Global Reporting Initiative (GRI). This procedure also included timing, tools, roles and responsibilities of the functions and individuals. To ensure responsiveness and proper application of the procedures, the reporting process was extensively discussed and agreed upon by the working group. The information presented in this report refers solely to 2021 and includes a comparison with the previous year or past where possible. The information collected and reported is based on measurable data. In addition, in order to correctly present the Group’s performance and help to ensure data reliability, the use of estimates has been limited as much as possible. If they are provided, they have been made using the best methods available and are properly identified. The statement presents both positive and negative aspects equally with a comment on the results when appropriate. This report was approved by the Board of Directors of Stevanato Group S.p.A. on April 11, 2022. This document was submitted to a compliance opinion (“limited assurance engagement” according to the criteria indicated by the ISAE 3000 Revised standard) by Deloitte & Touche S.p.A., which expresses, in a separate report, a certificate of compliance with the GRI Standards. The audit was carried out in accordance with the procedures indicated in the “Independent Auditors’ Report”, included in this document. For further information and suggestions regarding Stevanato Group Sustainability Report, please contact: su-stainability@stevanatogroup.com. VIII |

Stevanato Group1

Sustainability Report—2021 1.1 Highlights Stevanato Group 10 |
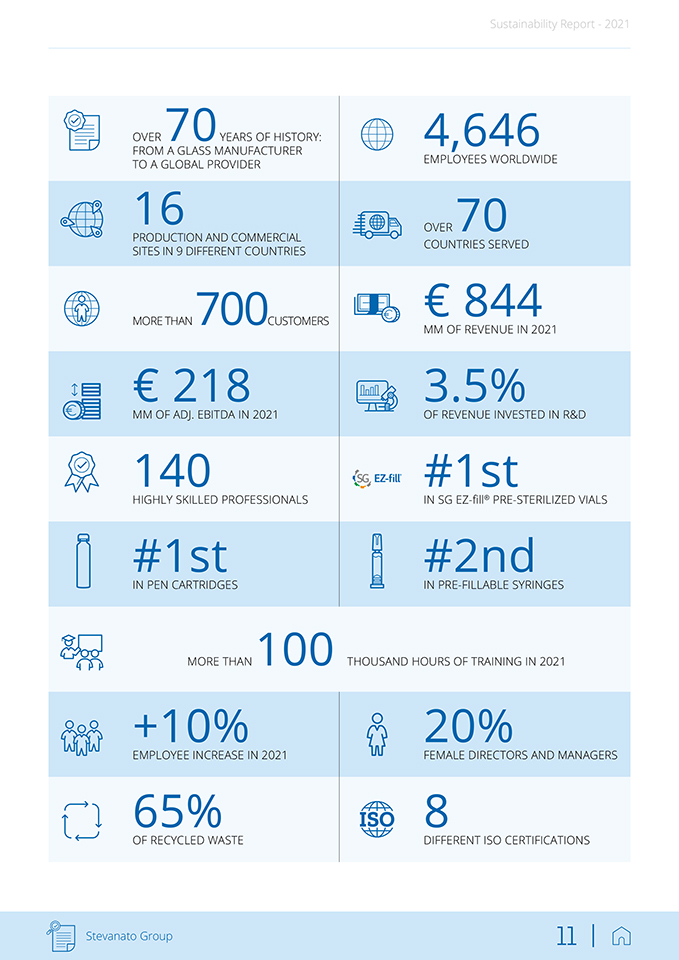
Sustainability Report—2021 OVER 70YEARS OF HISTORY: FROM A GLASS MANUFACTURER TO A GLOBAL PROVIDER 16 PRODUCTION AND COMMERCIAL SITES IN 9 DIFFERENT COUNTRIES MORE THAN 700CUSTOMERS € 218 MM OF ADJ. EBITDA IN 2021 140 HIGHLY SKILLED PROFESSIONALS #1st IN PEN CARTRIDGES MORE THAN 100 +10% EMPLOYEE INCREASE IN 2021 65% OF RECYCLED WASTE 4,646 EMPLOYEES WORLDWIDE OVER 70 COUNTRIES SERVED € 844 MM OF REVENUE IN 2021 3.5% OF REVENUE INVESTED IN R&D #1st IN SG EZ-fill® PRE-STERILIZED VIALS #2nd IN PRE-FILLABLE SYRINGES THOUSAND HOURS OF TRAINING IN 2021 20% FEMALE DIRECTORS AND MANAGERS 8 DIFFERENT ISO CERTIFICATIONS Stevanato Group 11 |

Sustainability Report—2021 1.2 Story and evolution Stevanato Group, an Italian multinational company, is a leading global supplier of drug containment, delivery and diagnostic solutions to the pharmaceutical, biotechnology and life sciences industries. OWNERSHIP AS OF 31.12.2021 78,03% Stevanato 11,17% Holding S.r.l. Stevanato Group S.p.A. (Market shares) 10,43% Stevanato Group S.p.A. (Treasury shares) 0,37% Shares Owned by Directors and Officers Stevanato Group 12 |

Sustainability Report—2021 Headquartered in Piombino Dese (Padua, Italy), Stevanato Group is a joint stock company with a total of € 21.698.480 in paid-up capital. The majority shareholder is Stevanato Holding S.r.l. with 78.03% of shares owned, followed by Stevanato Group S.p.A. with 10.43% of shares held in treasury, other shareholders hold 11.17% of share capital and, lastly, a group of directors and officers with 0.37% of shares owned. In 1949, Giovanni Stevanato founded Soffieria Stella, a specialty glass manufacturer in Zelarino, near Venice. Soffieria Stella, the precursor to Steva-nato Group, operated until 1959 when Stevanato Group was established in Piombino Dese (Padua). Over the last 70 years, Stevanato has evolved from an Italian glassware manufacturer to a leading global provider of integrated solutions for the healthcare industry. Stevanato Group delivers an integrated, end-to-end portfolio of products, processes and services that address customer needs across the entire drug life cycle at each of the development, clinical and commercial stages. Reduced time Reduced supply Reduced total Consistent to market chain risk cost of ownership quality Unique combination of integrated capabilities to address customer needs across the pharmaceutical value chain Drug containment solutions Highly Customized and Pre-Sterilized Containment Solutions (SG EZ-fill®) through Ompi brand Drug Delivery Systems Proprietary Devices and CMO/CDMO Business Technologies & Manufacturing Equipment Sub & Final Assembly, Automation, & Packaging Solutions, Visual Inspection, Glass Converting Analytical Services & Regulatory Support (incl. TECs) Analytical Testing & Regulatory Support Systems Processes Services The Group’s priority is to provide flexible solutions that preserve the integrity of pharmaceutical products and enable its customers to deliver safe and effective treatments to patients while reducing time to market, total cost of ownership (i.e., logistics, drug product waste, storage and personnel costs) and supply chain risk. Steva-nato Group achieves this by developing products in close collaboration with its customers and leveraging its scientific research capabilities, technical expertise and engineering and manufacturing excellence to meet their quality requirements. Stevanato Group 13 |

Sustainability Report—2021 Stevanato Group solutions are highly integrated with the development, production and commercialization processes of customers. In addition to manufacturing drug containment and delivery solutions, Stevanato provides a full set of services across all stages of drug development, from pre-clinical to clinical and commercialization. The Group also engineers machinery and equipment for the production of drug containment and delivery systems that can be integrated into both its customers’ and its own manufacturing processes. The Group’s involvement at each stage of a drug’s life cycle, together with the breadth of Stevanato’s offerings, enables it to serve as a one-stop-shop for customers, which gives Stevanato Group a significant competitive advantage. The chart below illustrates the Group’s presence across the pharmaceutical value chain. Stevanato Group operates across the healthcare industry and serves some of its fastest-growing segments, including biologics, biosimilars, vaccines and molecular diagnostics. The Group is embedded in the drug production and delivery supply chain and well-positioned to benefit from secular trends within its targeted focus areas, such as increases in demand resulting from pharmaceutical innovation, acceleration and expansion of vaccination programs, growth of biologics/biosimilars, self-administration of medicines, aging demographics, increasing complexities in health conditions and co-morbidities, and evolving quality standards and regulation. Stevanato Group 14 |
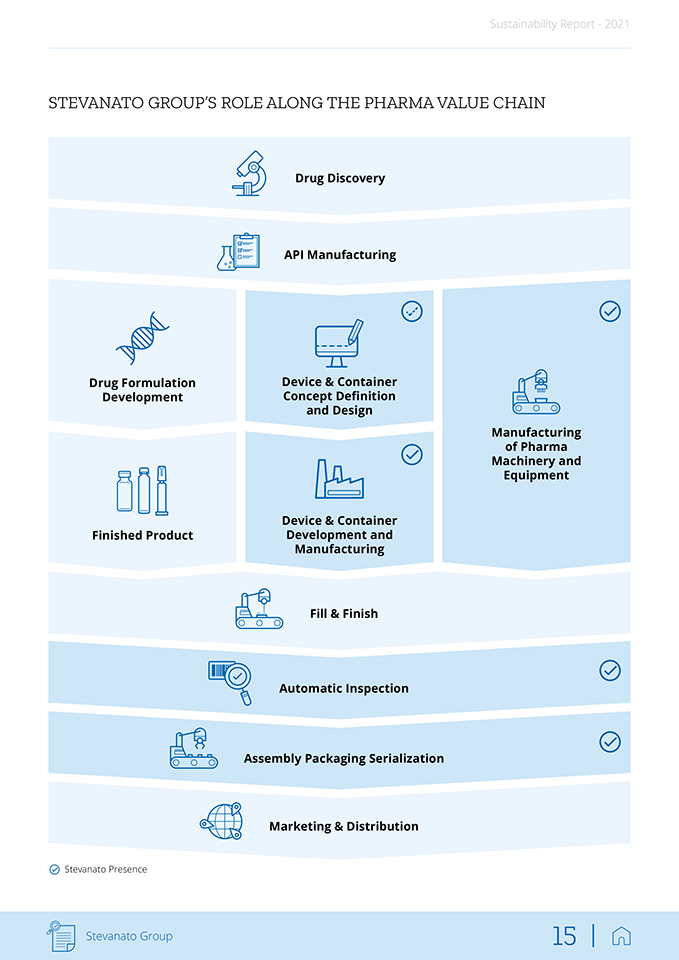
Sustainability Report—2021 STEVANATO GROUP’S ROLE ALONG THE PHARMA VALUE CHAIN Drug Discovery API Manufacturing Drug Formulation Development Device & Container Concept Definition and Design Manufacturing of Pharma Machinery and Equipment Finished Product Device & Container Development and Manufacturing Fill & Finish Automatic Inspection Assembly Packaging Serialization Marketing & Distribution Stevanato Presence Stevanato Group 15 |
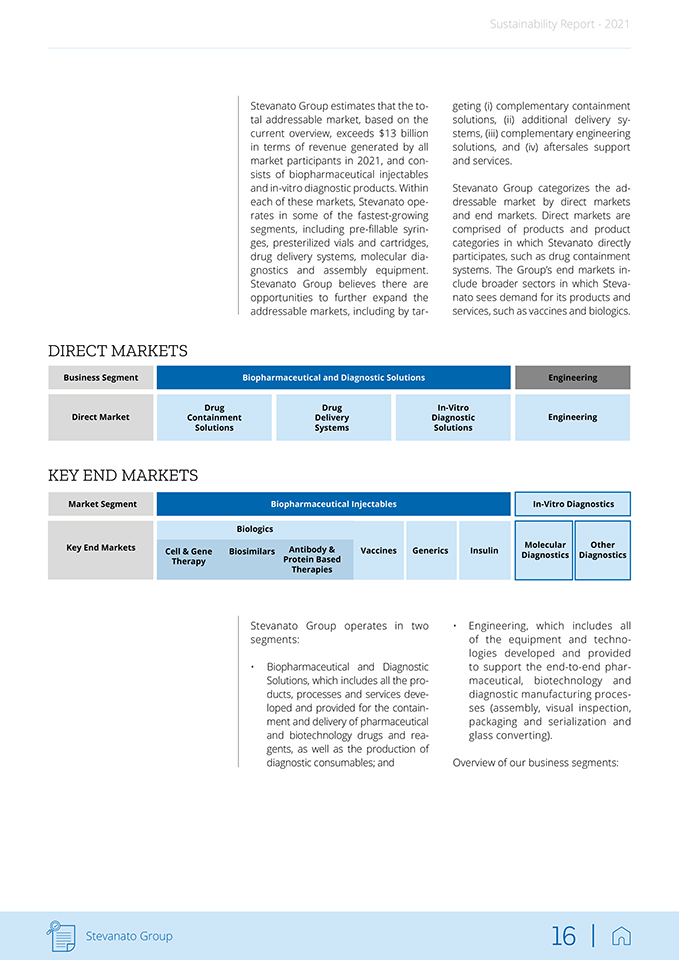
Sustainability Report—2021 Stevanato Group estimates that the to¬tal addressable market, based on the current overview, exceeds $13 billion in terms of revenue generated by all market participants in 2021, and con¬sists of biopharmaceutical injectables and in-vitro diagnostic products. Within each of these markets, Stevanato ope¬rates in some of the fastest-growing segments, including pre-filiable syrin¬ges, presterilized vials and cartridges, drug delivery systems, molecular dia¬gnostics and assembly equipment. Stevanato Group believes there are opportunities to further expand the addressable markets, including by tar¬geting (i) complementary containment solutions, (ii) additional delivery sy¬stems, (iii) complementary engineering solutions, and (iv) aftersales support and services. Stevanato Group categorizes the ad-dressable market by direct markets and end markets. Direct markets are comprised of products and product categories in which Stevanato directly participates, such as drug containment systems. The Group’s end markets in-clude broader sectors in which Steva¬nato sees demand for its products and services, such as vaccines and biologies. DIRECT MARKETS Business Segment Engineering Drug Drug In-Vitro Direct Market ContainmentDeliveryDiagnostic Engineering SolutionsSystemsSolutions KEY END MARKETS Market Segment Biopharmaceutical Injectables In-Vitro Diagnostics Molecular Other Diagnostics Diagnostics Stevanato Group operates in two segments: Biopharmaceutical and Diagnostic Solutions, which includes all the pro-ducts, processes and services deve-loped and provided for the contain-ment and delivery of pharmaceutica and biotechnology drugs and rea-gents, as well as the production of diagnostic consumables; and • Engineering, which includes all of the equipment and techno¬logies developed and provided to support the end-to-end phar¬maceutical, biotechnology and diagnostic manufacturing proces¬ses (assembly, visual inspection, packaging and serialization and glass converting). Overview of our business segments: it ED Stevanato Group 16 |

Sustainability Report—2021 Biopharmaceutical and Diagnostic Solutions Segment Drug Containment Solutions Platforms for Platforms for syringers vials Platforms for Integrated cartridges syringe safety systems Drug Delivery Systems Auto- Pen Injectors Injectors Wearable Dry Powder Drug Delivery Inhalers Devices IVD Solutions Point-of-lab Point-of-Care Consumables Consumables Handheld Devices Analytical Services • Primary Container • Compatibility and Functionality with Drug Product • Container Closure Integrity • Medical Devices/ Combination Products • Developmental (non-GMP) Fill & Finish services • Consultancy (regulatory, compilance support, test method development and transfer) Offering Proprietary “High-Value Solutions” Across Business Lines Over the past decades, Stevanato’s growth has been driven by cutting-edge developments in new containment and delivery solutions as well as strategic acquisitions. In 2005, the Group began its international expansion with the acquisition of Medical Glass, a Slovakia-based primary packaging manufacturing company. Subsequently, in 2007 and 2013, Stevanato acquired an Italian company, Optrel, and a Danish company, Inno-scan, which specialize in the production of inspection machines. These acquisitions marked its entry into the techno- logy and equipment manufacturing business. In 2016, Stevanato furthered its expansion through the acquisition of: (i) Balda, a company specialized in developing and manufacturing plastic diagnostic consumables, drug delivery systems and medical components; (ii) SVM, a company specialized in the production of high-tech machines and systems for assembly, packaging and serialization of pharmaceutical products; and (iii) Medirio, a start-up developing patents and other intellectual property for the wearable injectors business. Stevanato Group 17 |

Sustainability Report—2021 Engineering Segment Assembly • Sub-assembly and final assembly for drug delivery devices • Highly automated modular platforms Visual Inspection • Semi-automatic and automatic inspection machines • Diversifield portfolio • Artificial Intelligence Platform for AVI Packaging & Serialization • Single point of contact for packaging processes • Range of formats, reproducible settings • Traceability with serialization Glass Converting • Fully automated high-speed precision glass converting lines • Extensive dimensional and cosmetic inline controls After-Sales • Advanced after-sales services • Global service based on technical expertise and interactive tools Highly automated and advanced equipment for both in-house use and sale to customers Stevanato Group 18 |
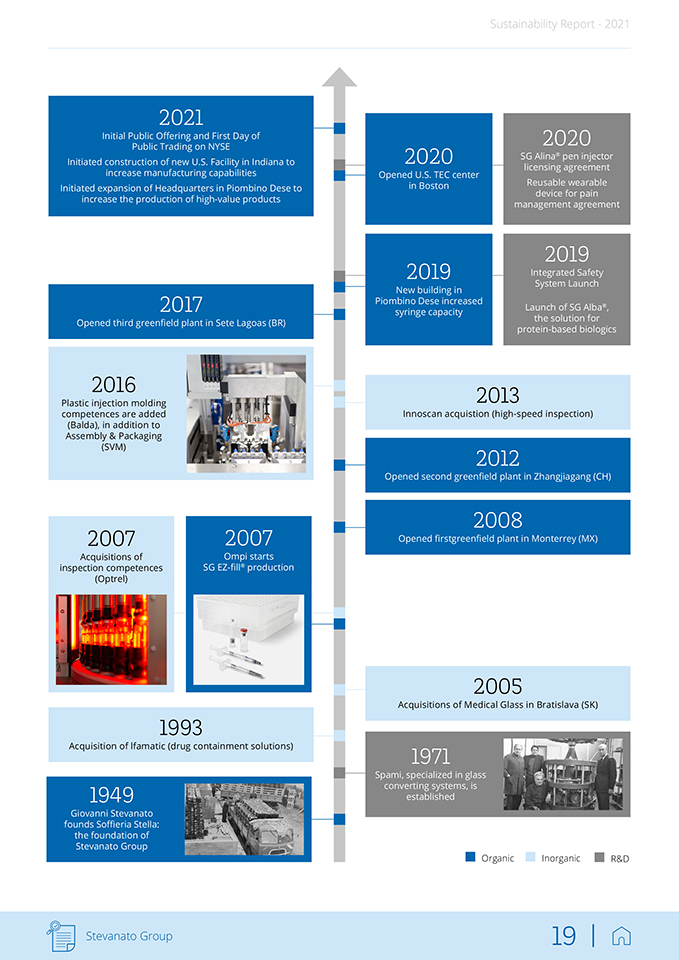
Sustainability Report—2021 2021 Initial Public Offering and First Day of Public Trading on NYSE Initiated construction of new U.S. Facility in Indiana to increase manufacturing capabilities Initiated expansion of Headquarters in Piombino Dese to increase the production of high-value products 2017 Opened third greenfield plant in Sete Lagoas (BR) 2016 Plastic injection molding competences are added (Balda), in addition to Assembly & Packaging (SVM) 2007 Acquisitions of inspection competences (Optrel) 2007 Ompi starts SG EZ-fill® production 1993 Acquisition of lfamatic (drug containment solutions) 1949 Giovanni Stevanato founds Soffieria Stella: the foundation of Stevanato Group 2020 Opened U.S. TEC center in Boston 2019 New building in Piombino Dese increased syringe capacity 2020 SG Alina® pen injector licensing agreement Reusable wearable device for pain management agreement 2019 Integrated Safety System Launch Launch of SG Alba®, the solution for protein-based biologics 2013 Innoscan acquistion (high-speed inspection) 2012 Opened second greenfield plant in Zhangjiagang (CH) 2008 Opened firstgreenfield plant in Monterrey (MX) 2005 Acquisitions of Medical Glass in Bratislava (SK) 1971 Spami, specialized in glass converting systems, is established Organic Inorganic R&D Stevanato Group 19 |

Sustainability Report—2021 In parallel with Stevanato’s acquisition strategy, the Group regularly reviews its operations in the context of its organic growth plan. As a result of these ongoing assessments, Stevanato Group has expanded its offering through new departments, new laboratories, new offices and new plants. In 2019, Stevana-to opened a new building in Piombino Dese (Italy) to increase its syringe production capacity, and since 2008, the Group has opened three greenfield sites in (i) Monterrey, Mexico in 2008; (ii) Zhangjiagang, China in 2012; and (iii) Sete Lagoas, Brazil in 2017. In 2021, the Group announced plans to expand its global market position in high-value solutions by investing in additional capacity in the U.S. and China to meet the growing demands of the expanding pharmaceutical, biotech-nology and vaccine markets. The site in Fishers, Indiana, USA, will enable the Group to better serve its North America pharmaceutical customers with the production of pre-sterilized EZ-fill® syringes and vials. The capacity expansion for EZ-fill® products in Zhangjiagang, China, also has a strong focus on biologics and vaccines in addition to focusing on solutions for the engineering segment. Another capacity expansion is planned in Italy where it will generate new jobs. The Group will follow best practices to improve both environmental and societal impacts using the latest in technology and sustainable solutions. Finally, in 2021 Stevanato Group finalized the consolidation of its plastic component operations in the Balda, Ontario site. The expansion of the On-tario plant was carefully implemented, minimizing impact on the staff and customers’ production schedules, and will greatly improve its level of service, reduce time to market, and simplify supply chain and customer interfaces. This rich history of organic growth and strategic acquisitions has allowed Ste-vanato Group to create a comprehensive portfolio of integrated products, capabilities, technologies and services for the pharmaceutical and heal-thcare industries. The main company brands are: • Ompi – Drug Containment Solutions: with more than 70 years of experience, Ompi is specialized in the production of glass primary packaging for pharmaceutical use and collaborates with pharma companies to continually raise its quality standards and develop customized solutions. • Balda – IVD and Drug Delivery Systems Solutions: founded in 1908, Balda specializes in high-precision plastic injection molding for pharmaceutical and healthcare applications including diagnostic consumables, dispensing devices and medical components. Balda offers both contract manufacturing and development services. • Lab/TEC – Analytical Services: born in 2015, SG Lab Analytics holds a leading edge in scientific and technological expertise. It offers a wide range of analytical services related to glass primary packaging with a focus on analytical chemistry, material properties, and physical and mechanical performance. Inaugurated in Boston, USA, in 2020 the Technology Excellence Center (TEC) provides biopharma companies with integrated analytical services and effective project management to support their drug development activities from early phase to commercialization and lifecycle management. • Spami – Glass Converting Equipment: Since 1971, Spami has been the technological leader in designing and assembling automated lines for the transformation of glass tubing into containers for pharmaceutical use. It continuously upgrades its technologies, addressing the real needs of glass tubing packaging manufacturers, offering higher quality, stability, output speed and facility of use. Stevanato Group 20 |

Sustainability Report—2021 • Optrel – Visual Inspection Equipment: since it was founded in 1983 in Vicenza, Italy, Optrel has been developing advanced inspection technologies for pharmaceutical products. The company focuses on the inspection machine market for the pharmaceutical industry, including parenteral drugs, injectables and solid dosage inspection using medium-speed automatic, semiauto-matic and manual equipment, supporting pharma companies from the lab to commercialization • Innoscan – Visual Inspection Equipment: founded in 1988, the company is a Danish technology leader in the development, production and marketing of complex, high-speed inspection machines for difficult-to-inspect pharmaceutical injectables. It is specialized in the field of automatic equipment designed to cater to pharma companies’ needs for high-volume production and holds a significant track-record in the delivery of machines optimized to inspect insulin for diabetes treatment. SVM – Assembly & Packaging Equipment: founded in 1974 in Denmark, SVM is specialized in the design and construction of high-technology machines and systems for assembly, secondary packaging and serialization for pharmaceutical and combination products. The company develops specialized machines from the conceptual phase to production. Stevanato Group 21 |

Sustainability Report—2021 The combination of multinational a truly global company spread across dif-brands has transformed the Group into ferent countries and continents: 9 production plants for manufacturing and assembly of pharmaceutical and healthcare products across the world: • Drug containment solutions: • Italy – Piombino Dese (PD), Latina (LT); • Slovakia – Bratislava; • Mexico – Monterrey; • Brazil – Sete Lagoas; • China – Zhangjjagang; • IVD and Drug Delivery Systems Solutions: • Germany – Bad Oeynhausen; • USA – Ontario (CA), Oceanside (CA)1. 5 plants for the engineering and production of technologies and manufacturing equipment: • Glass Converting: • Italy – Piombino Dese (PD), Bologna; • Visual Inspection: • Italy – Piombino Dese (PD); • Denmark – Brabrand; • Assembly & Packaging: • Italy – Piombino Dese (PD), Bologna; • Denmark – Silkeborg. 2 sites for analytical services: • Italy – Piombino Dese (PD); • USA – Boston (MA). 2 commercial offices: • Japan – Nagoya; • USA – Newton (PA). 2 sites under construction: • USA – Fishers (IN); • China – Zhangjjagang. The manufacturing facilities in Mexico, China, Brazil and the hub under construction in the US all represent gre-enfield operations built by the Group. Facilities in Slovakia, Germany, Den-mark and the United States (the plant dedicated to IVD and DDS Solutions) were acquired in strategic transactions over the past 15 years. Our global presence, together with proprietary, standardized manufacturing systems and processes, allow the Group to provide consistent product and services standards to its customers in approximately 70 countries around the world. 1. Balda Precision Inc., Production metal components. Stevanato Group 22 |
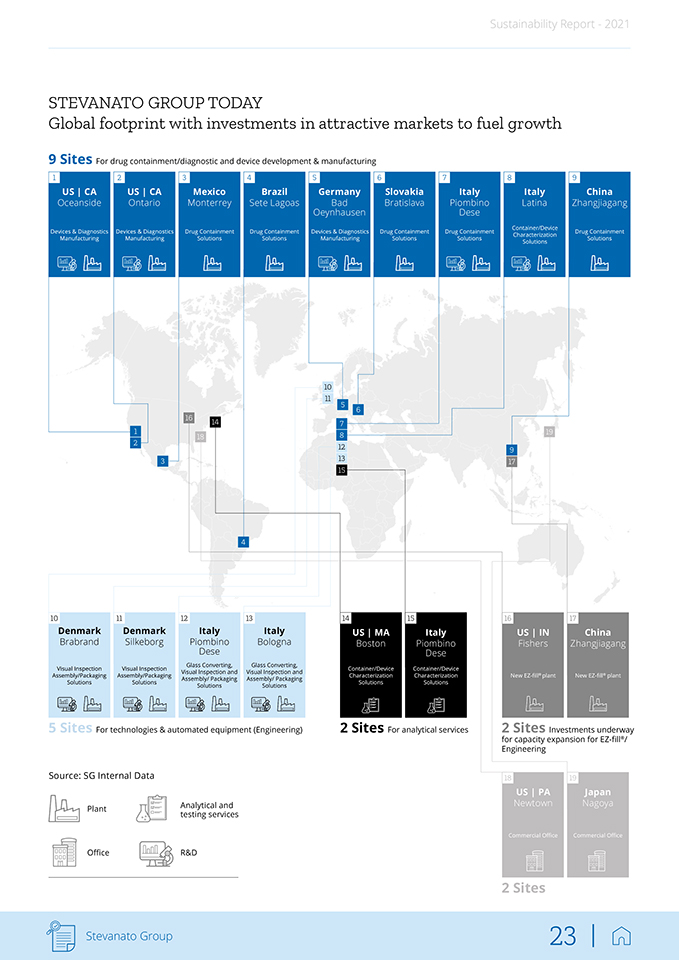
Sustainability Report—2021 STEVANATO GROUP TODAY Global footprint with investments in attractive markets to fuel growth 9 Sites For drug containment/diagnostic and device development & manufacturing 1 2 3 4 5 6 7 8 9 US | CA US | CA Mexico Brazil Germany Slovakia Italy Italy China Oceanside Ontario Monterrey Sete Lagoas Oeynhausen Bad Bratislava Piombino Dese Latina Zhangjiagang Container/Device Devices & Diagnostics Devices & Diagnostics Drug Containment Drug Containment Devices & Diagnostics Drug Containment Drug Containment Drug Containment Characterization Manufacturing Manufacturing Solutions Solutions Manufacturing Solutions Solutions Solutions Solutions 10 11 5 6 16 14 7 1 19 18 8 2 12 9 3 13 17 15 4 10 11 12 13 14 15 16 17 Denmark Denmark Italy Italy US | MA Italy US | IN China Brabrand Silkeborg Piombino Dese Bologna Boston Piombino Fishers Zhangjiagang Dese Glass Converting, Glass Converting, Visual Inspection Visual Inspection Container/Device Container/Device Visual lnspection and Visual lnspection and ® ® Assembly/Packaging Assembly/Packaging Characterization Characterization New EZ-fill plant New EZ-fill plant Assembly/ Packaging Assembly/ Packaging Solutions Solutions Solutions Solutions Solutions Solutions 5 Sites For technologies & automated equipment (Engineering) 2 Sites For analytical services 2 Sites Investments underway for capacity expansion for EZ-fill®/ Engineering Source: SG Internal Data 18 19 US | PA Japan Analytical and Newtown Nagoya Plant testing services Commercial Office Commercial Office Office R&D 2 Sites Stevanato Group 23 |

Sustainability Report—2021 1.3 Mission, Vision and Values Stevanato Group’s Vision, Mission and Values provide a framework to guide the company’s pursuit of business goals with an ethical and transparent mindset and focus on fostering innovation. Stevanato Group 24 |

Sustainability Report—2021 MISSION The Group is committed to creating systems, processes and services that enhance the integrity of medicines. It strives to be the best, objective-focused partner in the research and delivery of innovative solutions to support the success of its customers. The continual innovation and pioneering of new trends in pharmaceutical glass packaging ensures the production of the world’s most advanced solutions year after year, enhancing the level of product integrity guaranteed to patients and always seeking to exceed customer expectations. Geographical Product Focus on customer Operational Quality portfolio and patient excellence Merges and Social Innovation Financial Research and acquisition development VISION & VALUES At the heart of Stevanato Group’s vision is the desire to drive technological innovations that combine pharmaceutical glass products and processes into systems, which then enhance the integrity of parenteral drugs in a way that is far superior to anything that has been achieved before. Stevanato Group’s vision is based on five fundamental values, each one closely connected to the other in order to guarantee synergistic collaboration and leadership rooted in excellence. Employees within the Group are particularly dedicated to being ethical and accountable in their day-to-day actions and are driven to deliver results. Listen and Trust and respect Be Deliver Be ethical communicate with everyone accountable results always transparency and honesty Stevanato Group 25 |

Sustainability Report—2021 GUIDING PRINCIPLES Guiding Principles provide practical direction on how to be more effective in living the company Values every day. They help Stevanato Group’s people to work as an aligned, integrated team in pursuit of the mutual goal of building value for the Group, with a focus on customer needs, best product development, and efficiency. Being humble, competent and ready to aim for excellence are the principles that have characterized Stevanato Group, helping it to become the corporation it is today. The adoption of the Guiding Principles leads the Group towards achieving its Mission and creates systems, processes and services that guarantee the integrity of medicines. Will to win Competence, Consistency well, have a Don’t be Humility experience and execution and big goal standard preparation decision making Stevanato Group 26 |

Sustainability Report—2021 1.4 The company structure and main corporate functions The organizational chart below shows the composition of the Group including its parent company, Stevanato S.p.A., and subsidiaries. For more detailed information on the Group structure, please refer to the Consolidated Financial Statements as of De-cember 31, 2021. The Group corporate structure as of December 31, 2021, was the following: Stevanato Group 27 |
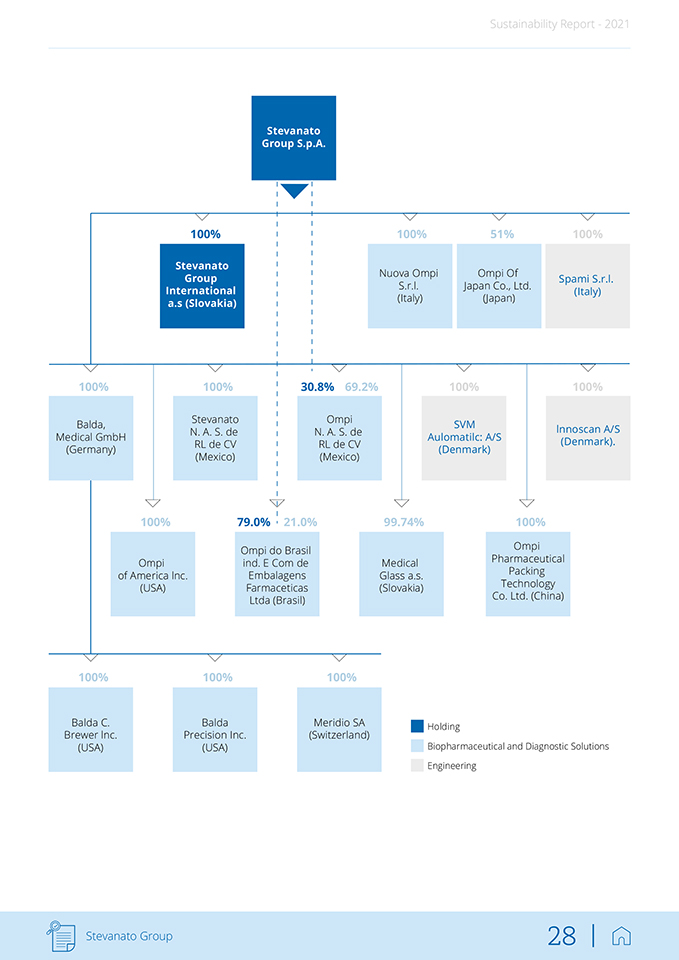
Sustainability Report—2021 Stevanato Group S.p.A. 100% 100% 51% 100% Stevanato Group Nuova Ompi Ompi Of Spami S.r.l. International S.r.l. Japan Co., Ltd. (Italy) a.s (Slovakia) (Italy) (Japan) 100% 100% 30.8% 69.2% 100% 100% Balda, Stevanato Ompi SVM N. A. S. de N. A. S. de lnnoscan A/S Medical GmbH Aulomatilc: A/S (Denmark). (Germany) RL de CV RL de CV (Denmark) (Mexico) (Mexico) 100% 79.0% 21.0% 99.74% 100% Ompi do Brasil Ompi Ompi ind. E Com de Medical Pharmaceutical of America lnc. Embalagens Glass a.s. Packing (USA) Farmaceticas (Slovakia) Technology Ltda (Brasil) Co. Ltd. (China) 100% 100% 100% Balda C. Balda Meridio SA Holding Brewer lnc. Precision Inc. (Switzerland) (USA) (USA) Biopharmaceutical and Diagnostic Solutions Engineering Stevanato Group 28 |

Sustainability Report—2021 In terms of significant changes to the organizational structure, as of February 22, 2021, Franco Moro has replaced Franco Stevanato as Chief Executive Officer of Stevanato Group, and is responsible for overseeing the execution of business development plans to grow and enhance the company’s end-to-end offering for pharmaceutical and diagnostics companies. Franco Moro previously held key Officer positions within Stevanato Group and has significant experience in international manufacturing and pharmaceutical industries. After this re-organization, Franco Steva-nato is the new Executive Chairman of the Board of Directors, overseeing long-term business strategy, acquisitions, expansion into global markets, and relations with the Board of Directors. Marco Stevanato remains the Vice-Chairman and Sergio Stevanato has been appointed Chairman of the Board Emeritus. Stevanato Group is proud of its heritage. The family’s close ties to the business have been fundamental in allowing the company to expand. It has been able to develop naturally while always understanding the ever-increasing complexity of doing business. As the Group has grown, a structured and skilled management team with international roots has been put into place. Stevanato Group has adopted a corporate governance that sets the rules for the appropriate management of the Group, separating ownership from the operating activities. The Group is managed by the Board of Directors, which meets at least four times per year to make key decisions on reserved topics. The Group is led by an experienced, highly motivated, diverse Board, leading to more objectivity and independence, and an executive team with a proven track record of operational excellence. The Group believes that this has significantly benefitted its strategy building and execution capability, helping it to consistently achieve results by anticipating market trends and staying ahead of the competition. Stevanato Group intends to continue with this approach. As of December 31, 2021, the Board of Directors was composed as follows: Board of Directors at 12.31.2021 Role Stevanato Sergio Chairman of the Board Emeritus Stevanato Franco Executive Chairman Stevanato Marco Vice-Chairman Moro Franco Chief Executive Officer Nicoletti Fabiano Independent Director Spinazzi Alvise Director Bonanni Fabrizio Independent Director Buttignon Fabio Independent Director Balachandran Madhavan Independent Director Morel Jr. Donald Eugene Independent Director Paola Vezzaro Independent Director William Federici Independent Director Stevanato Group 29 |
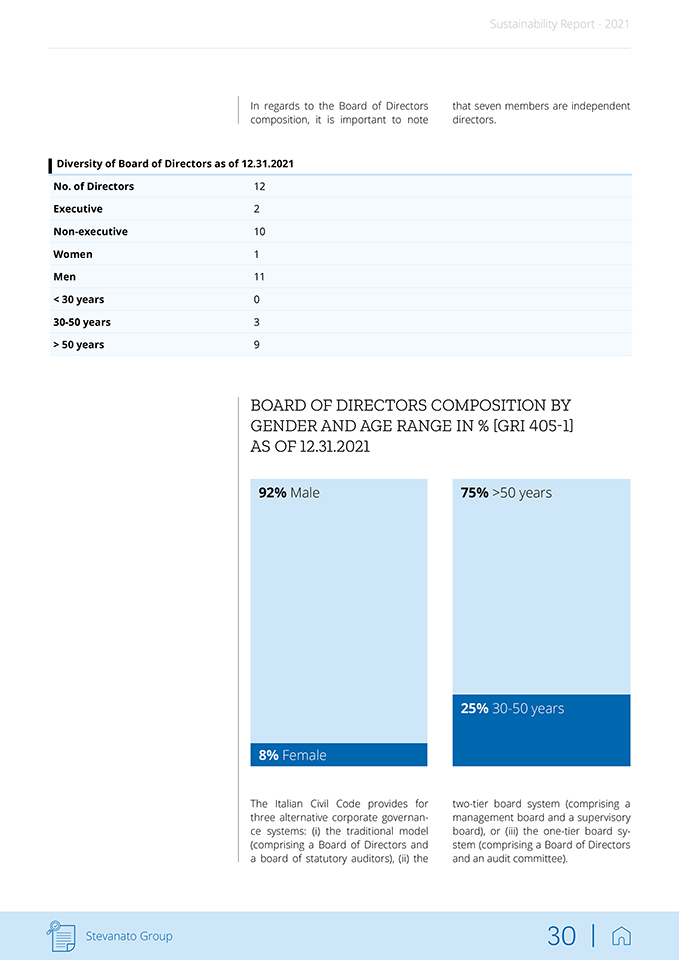
Sustainability Report—2021 In regards to the Board of Directors composition, it is important to note that seven members are independent directors. Diversity of Board of Directors as of 12.31.2021 No. of Directors 12 Executive 2 Non-executive 10 Women 1 Men 11 < 30 years 0 30-50 years 3 > 50 years 9 BOARD OF DIRECTORS COMPOSITION BY GENDER AND AGE RANGE IN % [GRI 405-1] AS OF 12.31.2021 92% Male 8% Female 75% >50 years 25% 30-50 years The Italian Civil Code provides for three alternative corporate governance systems: (i) the traditional model (comprising a Board of Directors and a board of statutory auditors), (ii) the two-tier board system (comprising a management board and a supervisory board), or (iii) the one-tier board system (comprising a Board of Directors and an audit committee). Stevanato Group 30 |

Sustainability Report—2021 In May 2021, Stevanato Group S.p.A. adopted the one-tier corporate governance system which provides for a Board of Directors and an Audit Committee. The Audit Committee consists of Wil-liam Federici, Fabio Buttignon and Fa-brizio Bonanni. Mr. Federici serves as the chairman of the Audit Committee. The Board of Directors determined that all members of the Audit Committee must meet the requirements for financial literacy under the applicable rules and regulations of the SEC and the NYSE corporate governance rules. The Audit Committee is compliant with applicable rules and regulations of the SEC and NYSE corporate governance rules as well as Italian Law requirements with respect to its composition, expertise requisites, functioning and independence. The Audit Committee is responsible for, among other things, assisting the board in the oversight of: • the accounting and financial reporting practices of the Company as well as the integrity of the financial statements; • the adequacy of the Company’s organizational structure, internal control system, and administrative and accounting systems; • the Company’s risk assessment and risk management processes to ensure such processes are effective; • the supervision of compliance with legal and regulatory requirements including, as required by the rules and regulations of the SEC, the preparation of the Audit Committee report to be included in the Company’s annual proxy statement; • the independence and qualifications of the Company’s registered public accounting firm. The Audit Committee meets in a manner that the Audit Committee may deem fit at least once every ninety calendar days. Periodically, the Audit Committee also meets with the independent auditor and members of management. A more detailed analysis of the governance structure indicates there are also four committees that cooperate with the Board of Directors on day-to-day matters. The members of the different committees described in this document share the same term of office with the Board of Directors and provide opinions and suggestions, without prejudice to the Board’s competence and decision-making responsibility. Below is the main composition and activities of the four Stevanato Group committees. Stevanato Group 31 |

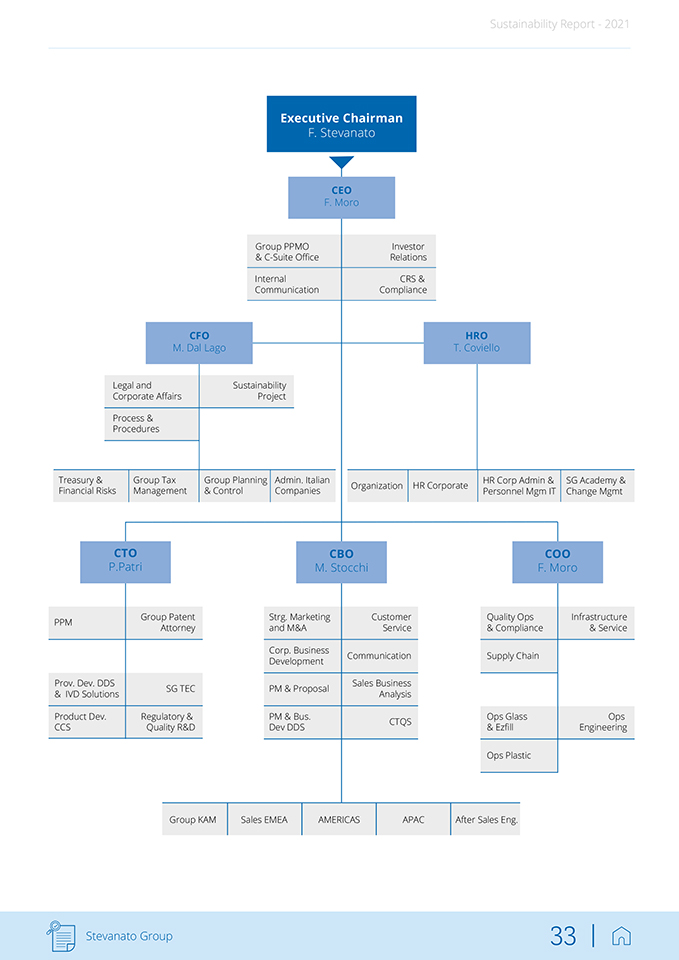
Sustainability Report—2021 The Nomination and Corporate Governance Committee is responsible for, among other things: Members Activities 4 members from the Board of • reviewing the structure, size and composition (including the skills, knowledge, Directors experience and diversity) of the Board of Directors; • identifying and appointing independent Board of Directors’ candidates to fill independent Board vacancies as and when these arise; • keeping under review the leadership needs of the organization, both executive and non-executive, with a view to ensuring the continuing ability of the organization to compete effectively in the marketplace; • constantly reviewing corporate governance rules and practices and ensuring that corporate governance codes that apply to the Company are observed; • formulating succession plans. The Business & Strategy Committee is responsible for, among other things: 8 members from the Board of • periodically reviewing and making recommendations on medium and long-term Directors strategies and strategic plans to be pursued; • reviewing the annual business plan, budget and capital structure of the Group before onward submission to the Board for approval; • meeting with management periodically to monitor the Company’s progress against its strategic goals and to discuss, review and recommend to the Board any such matters or issues which relate to the strategic planning of the Group; • ensuring the Board of Directors is regularly appraised of the Company’s progress with respect to implementation of any approved strategy. The Compensation Committee is responsible for, among other things: 3 members from the Board of • analyzing, discussing and making recommendations to the Board of Directors Directors on remuneration policies for directors and senior management and reviewing their appropriateness; • within the terms of the agreed policy and in consultation with the Board chairman, determining the total individual remuneration package of each Executive Director; • assessing, reviewing and recommending for approval by the Board, the CEO’s annual remuneration package and performance objectives based on the evaluation of the CEO’s performance; • reviewing and approving any significant changes to the overall compensation program and incentive plans. The ESG Committee is responsible for, among other things: 3 members from the Board of • assisting the Company in setting ESG strategies, including by reviewing, chal-Directors lenging and overseeing the content and approach to strategy concerning ESG matters; • supervising compliance of ESG disclosure and ensuring a sustainability strategy is considered by the Board as part of the overall business strategy of the Group; • bringing to the attention of the Board of Directors emerging ESG matters, and reviewing, challenging and approving annual sustainability KPIs and related targets in line with the agreed sustainability strategy; • putting systems in place to monitor ESG Matters and reviewing compliance with material regulation and legislation on ESG/sustainability issues, and any public ESG/sustainability-related commitments voluntarily subscribed to by the Group. The main corporate and business areas represent a significant investment for Stevanato and are essential to Ste-vanato Group’s operations and strategy. The purpose of the Group is to conduct a lawful, ethical, profitable and sustainable business in order to create value over the long term as reported below in the company chart. Stevanato Group 32 |
Sustainability Report—2021 Executive Chairman F. Stevanato CEO F. Moro Group PPMO Investor & C-Suite Office Relations Internal CRS & Communication Compliance CFO HRO M. Dal Lago T. Coviello Legal and Sustainability Corporate Affairs Project Process & Procedures Treasury & Group Tax Group Planning Admin. Italian HR Corp Admin & SG Academy & Organization HR Corporate Financial Risks Management & Control Companies Personnel Mgm IT Change Mgmt CTO CBO COO P.Patri M. Stocchi F. Moro Group Patent Strg. Marketing Customer Quality Ops Infrastructure PPM Attorney and M&A Service & Compliance & Service Corp. Business Communication Supply Chain Development Prov. Dev. DDS Sales Business SG TEC PM & Proposal & IVD Solutions Analysis Product Dev. Regulatory & PM & Bus. Ops Glass Ops CTQS CCS Quality R&D Dev DDS & Ezfill Engineering Ops Plastic Group KAM Sales EMEA AMERICAS APAC After Sales Eng. Stevanato Group 33 |

Sustainability Report—2021 1.5 Ethics, integrity and compliance An essential factor for success, and an indispensable asset to the Company’s reputation, is the adoption of high ethical conduct standards. The Values and Guiding Principles followed by the Group are the building blocks of its Code of Ethics. The Code of Ethics defines the guidelines and criteria of conduct for all recipients, and aims to ensure compliance with regulations in force to prevent improper acts or behavior and to help protect the legitimate interests of customers, employees, shareholders, business and financial partners, communities and all the different stakeholder groups. The Code of Ethics has been disseminated in all of Steva-nato Group’s companies. Overall, the Code of Ethics has the function of promoting fairness and loyalty in the management of transactions and human relations, within and outside the Group, by those who work on behalf of the Group. The recipients of the Code of Ethics, who are directors, employees, collaborators, suppliers and all those who, in any way, operate on behalf of the Group, are obliged to observe and enforce the provisions contained in it, by adopting the right behavior designed to preserve the Group’s image and the integrity of its economic and human heritage. Stevanato Group 34 |

Sustainability Report—2021 The specific provisions contained in the Code of Ethics have been reported in specific internal policies and procedures that ensure compliance with the principles and guidelines of the Code of Ethics (e.g., anti-corruption, insider trading, related parties and anti-discrimination). In this regard, it should be noted that the Group also approved an anti-bribery and anti-corruption policy on July 2, 2021, and approved an anti-discrimination policy on December 17, 2021. For further details on these policies please refer to the investors section of the corporate website. Stevanato Group acts in compliance with intellectual, industrial and commercial property rights, as well as with international laws and regulations in order to protect such rights. In 2021, no cases of non-compliance with environmental laws and regulations were registered. Since April 2021, Stevanato Group has adopted a whistleblowing procedure to correctly manage the reporting of any violations and irregularities concerning the Code of Ethics related to Italian Legal Entities. In this context, Stevanato Group ensures that business is conducted in line with fairness, completeness and transparency of information and legitimacy, excluding any form of corruption or favoritism. Employees can report any violations or suspected violations to the Company through a dedicated email address: csr-complian-ce@stevanatogroup.com. The information transmitted is promptly checked and, once the Report has been verified, the case is submitted to the competent corporate department for the application of any disciplinary sanctions or for the activation of contractual termination mechanisms. This whistleblowing procedure is designed to ensure the confidentiality of the person reporting the issue and the information received, as well as its validity. Note that, no grievances as of December 31, 2021, have been sent to the relevant function inbox and, during 2021, no cases of incidents of corruption or human rights violations were recorded in the Group. In accordance with Italian law, Steva-nato Group has introduced an Organizational, Management and Control model for its Italian entities as per Legislative Decree no. 231/01. The management principles envisaged by the Organizational, Management and Control framework are aimed at preventing the commission of crimes by employees, or any other crimes connected to Stevanato Group that may involve the Group’s Italian companies. The Group is currently working to strengthen management systems within the foreign companies to ensure compliance with each local law, taking into consideration the provisions of the Model of Organization, Management and Control pursuant to Legislative Decree 231/01. The parent company, Stevanato Group S.p.A., has set up a Supervisory Body pursuant to Legislative Decree 231/01, responsible for monitoring compliance, operating and updating the Model. A communication channel has been set up at the e-mail address odv.steva-natogroup@stevanatogroup.com, which only members of the Supervisory Board may access to receive reports of any violations or suspected violations of the Model from internal and external subjects. Stevanato Group 35 |

Sustainability Report—2021 A FOCUS ON STEVANATO GROUP RISK MANAGEMENT Risk monitoring and management is an integral part of Stevanato Group’s business model. Risk exposure is managed through careful monitoring carried out by each employee. At the moment, Stevanato Group tackles ESG risks & opportunities in accordance with current legislation and regulations. The main relevant risk categories identified are: • business risks related to activities and critical aspects of the business; • risks related to the efficiency and effectiveness of business operating processes with impact on Group performance; • risks related to human resources management and the effectiveness of the organizational structure; • risks related to financial planning processes and financial reporting activities, management of financial and insurance instruments; • risks related to compliance with national and international laws, regulations and Group policies. As proof of the Group’s ongoing commitment to ethical conduct and integrity in its business, there were no reports and/or complaints received about non-compliance with laws or regula- tions, or legal action taken regarding anti-competitive behavior, anti-trust or monopoly violations either in or out of court in 2021. Stevanato Group 36 |

Stevanato Group and sustainability SG Stevanato Group 2

Sustainability Report—2021| 2.1 Approach to sustainability Stevanato Group aims to align its corporate culture and business operations with relevant Environmental Social and Governance (ESG) elements pursuing a sustainable corporate model. The Group is committed to embedding sustainability values into its policies and practices and to establishing a culture of integrity. SG believes that the challenge and opportunity is to continue growing and supporting its customers through regenerative business innovation, while making a positive impact for the benefit of all, in everything the Company does. The Group considers itself an interdependent and responsible member of the community it operates in and, as such, promotes initiatives and solutions that foster the health and wellbeing of society and the planet as a whole. As a key player in the pharmaceutical and healthcare market, Stevanato Group is focused on developing and strengthening its sustainability practices. As stated in its EHS policy, Stevanato Group aims to protect the environment, to operate under the principles of sustainability on a global level, and to integrate EHS management into all business processes, including the commitment to energy-saving programs and the optimization of natural resources consumption. In addition, Stevanato Group pursues the Life Cycle Perspective of selected products, packaging and processes as part of its corporate Circular Economy innovation program. As such, it strives to improve the environmental and societal impact of its business through the integration of principles such as: (i) Design out waste and pollution; (ii) Keep products and materials in use; (iii) Regenerate natural systems. These sustainable practices can help mitigate impact in areas such as climate- change, biodiversity loss, and pollution. Finally, Stevanato Group fosters Public-Private co-innovation through collaboration with Universities/ Joint Labs to advance education and awareness on such topics. Through a materiality assessment, the Group has identified the ESG issues most relevant to the organization and its stakeholders. The issues considered have a substantial impact on Stevanato Group’s environmental, social and economic performance, or may substantially influence stakeholders’ decisions. Therefore, as required by the Standards of the Global Reporting Initiative (GRI), Stevanato Group has defined and organized the contents of the Sustainability Report to provide disclosure on how the Group manages sustainability by assessing these material issues. Sustainability 38
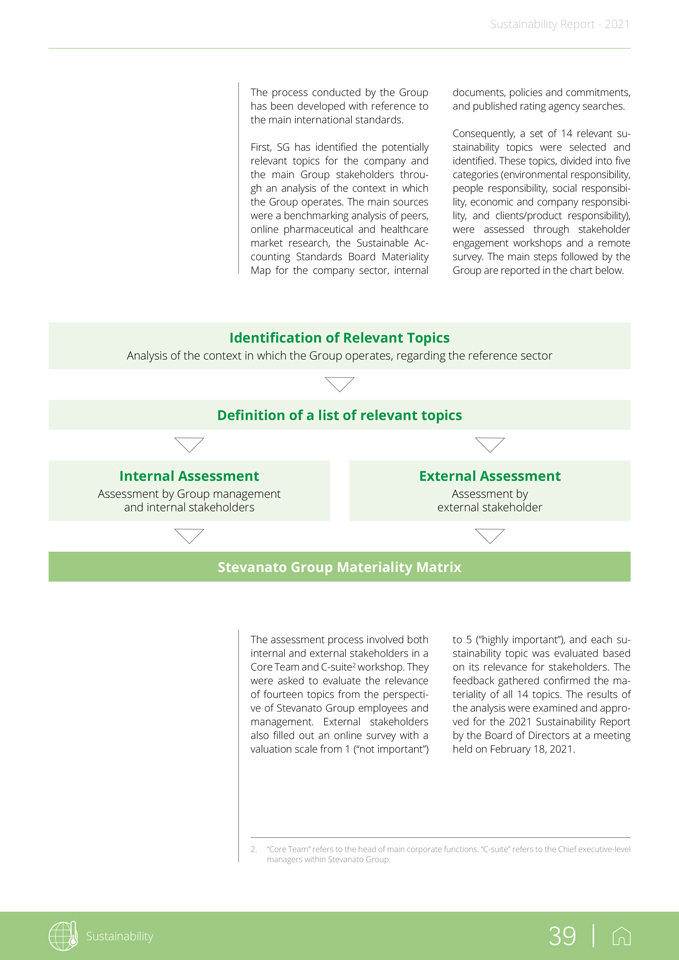
2. “Core Team” refers to the head of main corporate functions. “C-suite” refers to the Chief executive-level managers within Stevanato Group. Sustainability Report—2021 The process conducted by the Group has been developed with reference to the main international standards. First, SG has identified the potentially relevant topics for the company and the main Group stakeholders through an analysis of the context in which the Group operates. The main sources were a benchmarking analysis of peers, online pharmaceutical and healthcare market research, the Sustainable Accounting Standards Board Materiality Map for the company sector, internal documents, policies and commitments, and published rating agency searches. Consequently, a set of 14 relevant sustainability topics were selected and identified. These topics, divided into five categories (environmental responsibility, people responsibility, social responsibility, economic and company responsibility, and clients/product responsibility), were assessed through stakeholder engagement workshops and a remote survey. The main steps followed by the Group are reported in the chart below. Identification of Relevant Topics Analysis of the context in which the Group operates, regarding the reference sector Definition of a list of relevant topics Internal Assessment External Assessment Assessment by Group management Assessment by and internal stakeholders external stakeholder Stevanato Group Materiality Matrix The assessment process involved both internal and external stakeholders in a Core Team and C-suite2 workshop. They were asked to evaluate the relevance of fourteen topics from the perspective of Stevanato Group employees and management. External stakeholders also filled out an online survey with a valuation scale from 1 (“not important”) to 5 (“highly important”), and each sustainability topic was evaluated based on its relevance for stakeholders. The feedback gathered confirmed the materiality of all 14 topics. The results of the analysis were examined and approved for the 2021 Sustainability Report by the Board of Directors at a meeting held on February 18, 2021. Sustainability 39 |

Sustainability Report—2021 1 2 3 4 5 Research & Business ethics, Human capital Product quality Occupational Development governance and management and and responsibility Health & Safety and Innovation compliance development 14 6 Economic Local performance communities and value engagement MATERIAL TOPIC creation FOR SUSTAINABILITY REPORT 2021 13 7 Responsible GHG supply chain & Emissions procurement 12 11 10 9 8 Water Human Employee Waste Energy management rights wellbeing management Consumption The materiality assessment allowed the Group to identify 14 topics across five areas: environmental responsibility, people responsibility, social responsibility, economic and company responsibility and clients/product responsibility. These are considered highly relevant for Stevanato Group, as illustrated in the materiality matrix below. Sustainability 40 |
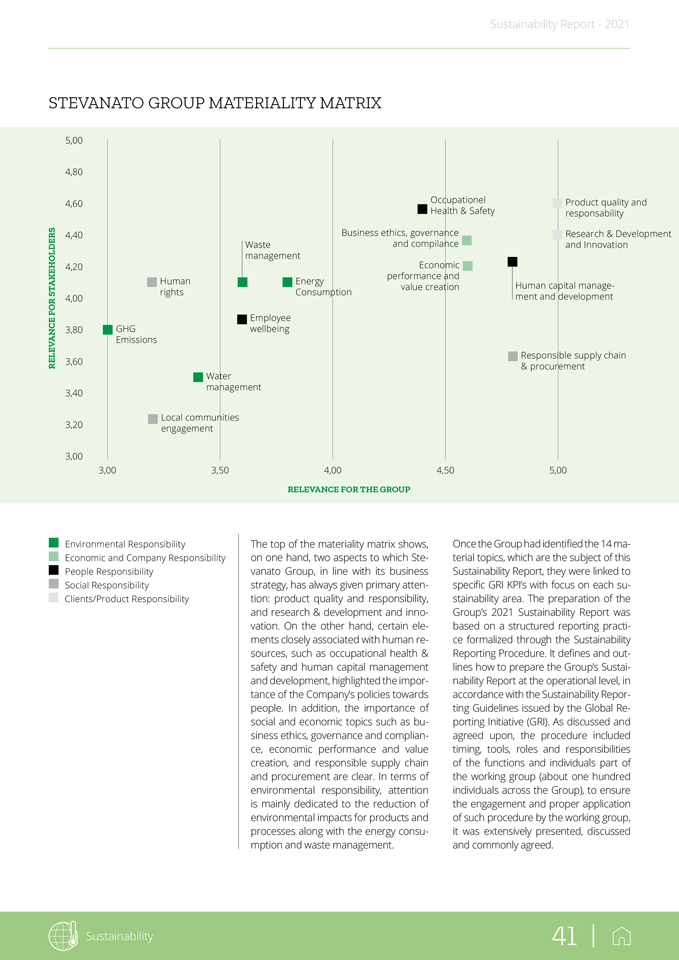
Sustainability 41 | Sustainability Report—2021 STEVANATO GROUP MATERIALITY MATRIX 5,00 4,80 4,60 Occupationel Product quality and Health & Safety responsability 4,40 Business ethics, governance Research & Development Waste and compilance and Innovation management Economic 4,20 performance and Human Energy Human capital manage-value creation STAKEHOLDERS rights Consumption FOR 4,00 ment and development Employee 3,80 GHG wellbeing Emissions Responsible supply chain RELEVANCE 3,60 & procurement Water management 3,40 Local communities 3,20 engagement 3,00 3,00 3,50 4,00 4,50 5,00 RELEVANCE FOR THE GROUP Environmental Responsibility Economic and Company Responsibility People Responsibility Social Responsibility Clients/Product Responsibility The top of the materiality matrix shows, on one hand, two aspects to which Ste-vanato Group, in line with its business strategy, has always given primary attention: product quality and responsibility, and research & development and innovation. On the other hand, certain elements closely associated with human resources, such as occupational health & safety and human capital management and development, highlighted the importance of the Company’s policies towards people. In addition, the importance of social and economic topics such as business ethics, governance and compliance, economic performance and value creation, and responsible supply chain and procurement are clear. In terms of environmental responsibility, attention is mainly dedicated to the reduction of environmental impacts for products and processes along with the energy consumption and waste management. Once the Group had identified the 14 material topics, which are the subject of this Sustainability Report, they were linked to specific GRI KPI’s with focus on each sustainability area. The preparation of the Group’s 2021 Sustainability Report was based on a structured reporting practice formalized through the Sustainability Reporting Procedure. It defines and outlines how to prepare the Group’s Sustainability Report at the operational level, in accordance with the Sustainability Reporting Guidelines issued by the Global Reporting Initiative (GRI). As discussed and agreed upon, the procedure included timing, tools, roles and responsibilities of the functions and individuals part of the working group (about one hundred individuals across the Group), to ensure the engagement and proper application of such procedure by the working group, it was extensively presented, discussed and commonly agreed.

Sustainability Report—2021 1 Product quality and responsibility 2 Research & Development and Innovation 3 Occupational Health & Safety 4 Business ethics, governance and compliance 5 Human capital management and development 6 Economic performance and value creation 7 Economic performance and value creation 8 Energy Consumption 9 Waste management 10 Employee wellbeing 11 Human rights 12 Water management 13 GHG Emissions 14 Local communities’ engagement Sustainability 42 |

tical issues emerged from stakeholder engagement activities carried out by Group companies. The following figure provides an overview of Stevanato’s seven most relevant stakeholder categories based on an in-depth analysis of the reference sector (peers and customers) carried out during the materiality analysis. Stevanato Group adopts practices that encourage dialogue and involvement with all stakeholder categories. Engagement is considered an essential element of the Group’s sustainability strategy and is directly correlated with the Group’s medium and long-term success. The main channels of dialogue and interaction are summarized below. The methods and frequency of stakeholder involvement vary according to the issues and opportunities subject to discussion during the year. Employes Shareholders and Board of Directors Regulators and authority Universities and research centres Community and local authority Customers Suppliers Finally, it is important to note that the procedure requires correct and compliant GRI KPIs associated with the involved functions and is coordinated by the Process Owner (Chief Financial Officer) and the Deputy Process Owner (responsible for the duties delegated by the Process Owner), as per assignment by the Board of Directors. Stevanato Group has also aligned its material topics with 15 of the 17 Sustainable Development Goals (SDGs) defined by the action program of the 193 UN member Countries. Below is a table showing the connection between Stevanato material topics and UN Sustainable Development Goals (SDGs). The Group has adopted flexible and diversified practices to share present and future Group development strategies with its main stakeholders. No cri-
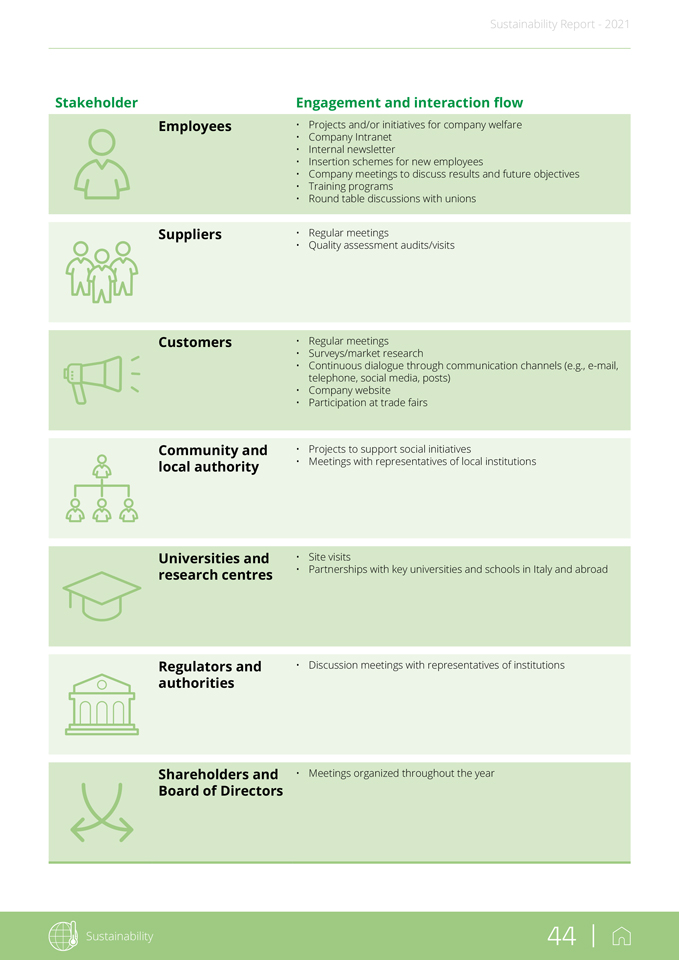
Sustainability Report—2021 Stakeholder Employees Suppliers Customers Community and local authority Universities and research centres Regulators and authorities Shareholders and Board of Directors Engagement and interaction flow • Projects and/or initiatives for company welfare • Company Intranet • Internal newsletter • Insertion schemes for new employees • Company meetings to discuss results and future objectives • Training programs • Round table discussions with unions • Regular meetings • Quality assessment audits/visits • Regular meetings • Surveys/market research • Continuous dialogue through communication channels (e.g., e-mail, telephone, social media, posts) • Company website • Participation at trade fairs • Projects to support social initiatives • Meetings with representatives of local institutions • Site visits • Partnerships with key universities and schools in Italy and abroad • Discussion meetings with representatives of institutions • Meetings organized throughout the year Sustainability 44 |

Sustainability Report—2021 2.2 Certifications and awards As part of SG’s continuous efforts to improve its processes, the Group aims to meet leading standards in the field, both in terms of industrial production and product sales, while minimizing the risks to the environment, health and safety in the workplace. This commitment is outlined in the following table, showing the Group’s compliance with the highest standards of certification. Sustainability 45 |
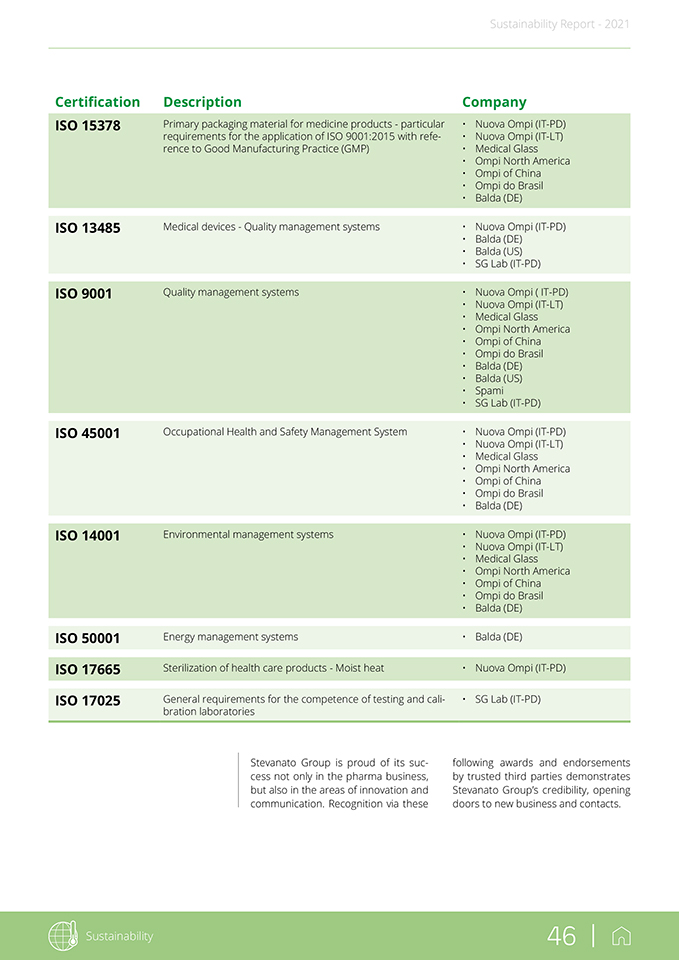
following awards and endorsements by trusted third parties demonstrates Stevanato Group’s credibility, opening doors to new business and contacts. Certification Description Company ISO 15378 Primary packaging material for medicine products—particular • Nuova Ompi (IT-PD) requirements for the application of ISO 9001:2015 with refe- • Nuova Ompi (IT-LT) rence to Good Manufacturing Practice (GMP) • Medical Glass • Ompi North America • Ompi of China • Ompi do Brasil • Balda (DE) ISO 13485 Medical devices—Quality management systems • Nuova Ompi (IT-PD) • Balda (DE) • Balda (US) • SG Lab (IT-PD) ISO 9001 Quality management systems • Nuova Ompi ( IT-PD) • Nuova Ompi (IT-LT) • Medical Glass • Ompi North America • Ompi of China • Ompi do Brasil • Balda (DE) • Balda (US) • Spami • SG Lab (IT-PD) ISO 45001 Occupational Health and Safety Management System • Nuova Ompi (IT-PD) • Nuova Ompi (IT-LT) • Medical Glass • Ompi North America • Ompi of China • Ompi do Brasil • Balda (DE) ISO 14001 Environmental management systems • Nuova Ompi (IT-PD) • Nuova Ompi (IT-LT) • Medical Glass • Ompi North America • Ompi of China • Ompi do Brasil • Balda (DE) ISO 50001 Energy management systems • Balda (DE) ISO 17665 Sterilization of health care products—Moist heat • Nuova Ompi (IT-PD) ISO 17025 General requirements for the competence of testing and cali- • SG Lab (IT-PD) bration laboratories Stevanato Group is proud of its success not only in the pharma business, but also in the areas of innovation and communication. Recognition via these

MOST IMPORTANT AWARDS ACHIEVED The Pharma Innovation Awards are a celebration of technologies contributing to advancements in risk reduction, product quality improvement and manufacturing efficiency. The prize awarded by Pharma Manufacturing acknowledges the investment pharmaceutical equipment vendors put into developing and improving their products. The award is a tribute to Stevanato Group’s ability to listen to the needs of the market and its customers, distinguishing itself as a leader in equipment and technology. In 2018, Pharma Manufacturing recognized that LDP (Low Delamination Propensity) Vials from Ompi (part of Stevanato Group), offered minimized delamination propensity, reducing the risk of drug product recalls. In 2021, Stevanato Group was recognized as a winner of Pharma Manufacturing’s Pharma Innovation Award for its Artificial Intelligence-ready (AI) Vision Robot Unit (VRU). A robotic human-like inspection unit, the VRU is a perfect match for the factory of the future, combining human-like flexibility with machine learning capabilities. In addition, SG Vision AI, Stevanato Group’s Artificial Intelligence-based platform for visual inspection machines, was nominated among the top technologies in the shortlist of Innovation Awards 2021 powered by “The Medicine Maker”. Contamination Lab, also present in the Veneto Region, is an open-innovation program that fosters entrepreneurial culture in young talent through the development of market-based innovative projects. In 2021, C_Lab hosted a competition in which 12 companies from different industries took part, including 230 candidates, 80 participants and 12 teams of talent. Stevanato Group supported a team as part of the corporate Circular Economy innovation program, where the purpose was to improve the environmental impact of glass-based End-Of-Life Drug Containment Solutions. The team won first prize in the competition for its achievement in identifying and creating upcycling concepts for the End Of Life of glass-based products. On August 19, 2021, the Slovak Chamber of Commerce and Industry awarded our Medical Glass plant in Slovakia for its contribution to economic development and business in the Slovak Republic. The award recognized how SG actively involves its stakeholders in the continuous improvement of processes focused on responsible development in the communities in which we operate. Launched in 1997 by Pharmapack, the Pharmapack Awards celebrate the latest innovations from packaging companies within the drug, medical device, health product and veterinary drug sectors. The Pharmapack Awards recognize innovations that have improved drug efficacy and user safety, or reduced environmental impact. In 2019, the cartridge-based wearable device designed by Stevanato Group won the prize for the Best Innovation in Drug Delivery Device.

Open Innovation is one of the pillars of the Smau company. The Innovation Awards are intended to be a recognition of companies and public bodies and aim to share and spread good practices. Stevanato Group received the award in 2019 for its “SYN Here” geo-mapping software designed by the I-Digital department. The intelligence platform mapped specific locations, internal/external spaces and objects in SG plants, building an interactive component into company information processes. By creating a virtual twin of the plant, the digital model helped shed light on critical infrastructure attributes such as compliance and safety issues.

2.3 Participation in organizations and associations Stevanato Group cooperates with an extensive network of trade associations and organizations, facilitating the exchange of ideas, knowledge and different points of view.

Founded in 1890, the Drug, Chemical & Associated Technologies Association, Inc. (DCAT) is a not-for-profit, corporate member-supported and volunteer-led global business development association for companies engaged in the Bio/Pharmaceutical manufacturing value chain. DCAT has been providing a life-long collegial community for its Member Companies and their representative for over 130 years. DCAT creates objective, expert-driven education programs and informational services, so its Member Companies can easily gain insight into industry market trends, best practices and the issues affecting the industry. The Parenteral Drug Association (PDA) is the leading global provider of science, technology and regulatory information. The PDA creates awareness and understanding of important issues facing the pharmaceutical and biopharmaceutical community and delivers high-quality, relevant education to the industry. Since its founding in 1946 as a nonprofit organization, PDA has been committed to developing scientifically sound, practical technical information and expertise to advance pharmaceutical/biopharmaceutical manufacturing science and regulation so members can better serve patients. The International Society for Pharmaceutical Engineering is the world’s largest not-for-profit association leading scientific, technical and regulatory advancement throughout the entire pharmaceutical lifecycle. ISPE is the global industry leader in connecting pharmaceutical knowledge to manufacturing and supply chain innovation, operational excellence and regulatory insights to enhance industry efforts to develop, manufacture and reliably deliver quality medicines to patients. MassBio is an American not-for-profit organization founded in 1985 that represents and provides services and support for the #1 life sciences cluster in the world. Mas-sBio’s mission is to advance Massachusetts’ leadership in the life sciences to grow the industry, add value to the healthcare system and improve patient lives. They represent the premier global life sciences and healthcare hub, with 1,400+ members dedicated to preventing, treating, and curing diseases through transformative science and technology that brings value and hope to patients. Founded in 1996, the Massachusetts Medical Device Industry Council (MassME-DIC) is the largest regional MedTech association in the United States with over 300 members. MassMEDIC is the primary source of advocacy, information and support to the region’s MedTech manufacturers, suppliers and associated non-profit groups. It advocates for sound public policy that supports innovation and fosters a community built on a shared purpose: saving and improving the lives of patients everywhere through medical technology.

The ICG (International Commission on Glass) is a non-profit international Society of national scientific and technical organizations with particular interest in glass science and technology. It was founded in 1933 and has grown to become the recognized worldwide organization in the field of glass with 37 current members, bringing together the world’s most respected universities, scientific institutions and companies in the glass industry. The aim of ICG is to promote and stimulate understanding and cooperation between glass experts in the fields of science and technology as well as art, history and education. The China National Pharmaceutical Packaging Association (CNPPA) is a national non-profit organization, formerly known as the National Pharmaceutical Packaging Technology Center. It was set up in Guangzhou in June 1980 and was officially renamed China National Pharmaceutical Packaging Association in November 1991 with the approval of the Ministry of Civil Affairs. The association has nearly 500 members.

Economic sustainability 3

Economic sustainability 3.1 Stakeholder value creation The creation and distribution of direct economic value demonstrates how Stevanato Group has created wealth for its stakeholders, as shown by the figures in the Direct economic value generated and distributed (EVG&D) chart below. Founded on an accrual basis, it includes the basic components from Profit and Loss (P&L) statements for the global operations in accordance with GRI Disclosure. Throughout Stevanato’s operational activities, the Group creates value for a wide variety of stakeholders, including: • Suppliers in terms of operating costs; • Personnel by considering employee wages and benefits as total payroll; • Lenders looking at financial charges; • Public administration with payments to governments such as taxes; • Communities including donations, sponsorships and collaboration.
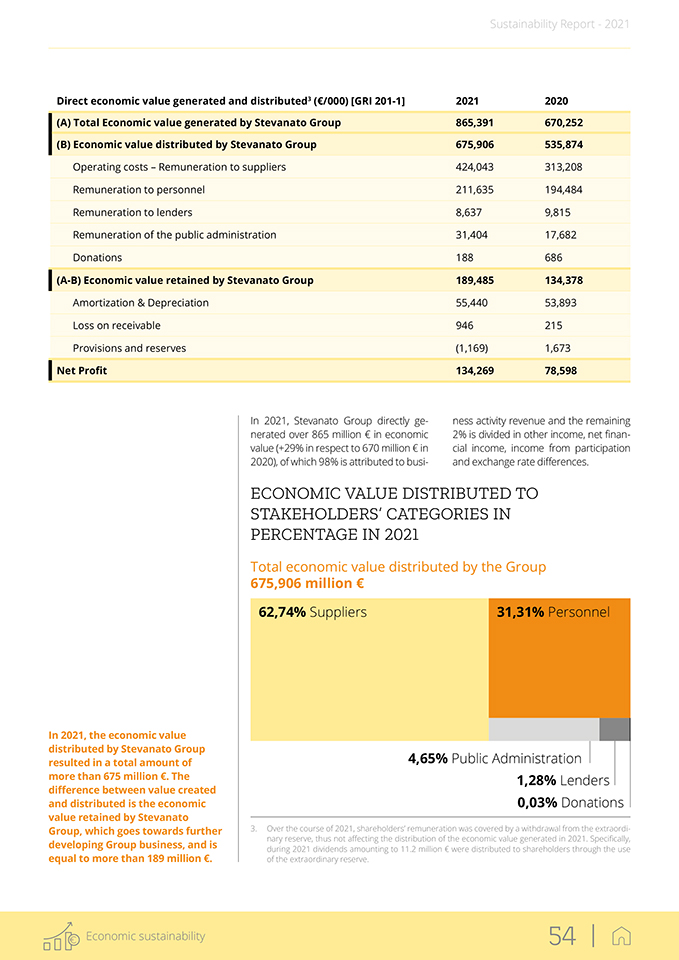
Direct economic value generated and distributed3 (€/000) [GRI 201-1] 2021 2020 (A) Total Economic value generated by Stevanato Group 865,391 670,252 (B) Economic value distributed by Stevanato Group 675,906 535,874 Operating costs – Remuneration to suppliers 424,043 313,208 Remuneration to personnel 211,635 194,484 Remuneration to lenders 8,637 9,815 Remuneration of the public administration 31,404 17,682 Donations 188 686 (A-B) Economic value retained by Stevanato Group 189,485 134,378 Amortization & Depreciation 55,440 53,893 Loss on receivable 946 215 Provisions and reserves (1,169) 1,673 Net Profit 134,269 78,598 In 2021, the economic value distributed by Stevanato Group resulted in a total amount of more than 675 million €. The difference between value created and distributed is the economic value retained by Stevanato Group, which goes towards further developing Group business, and is equal to more than 189 million €. In 2021, Stevanato Group directly generated over 865 million € in economic value (+29% in respect to 670 million € in 2020), of which 98% is attributed to busi- ness activity revenue and the remaining 2% is divided in other income, net financial income, income from participation and exchange rate differences. ECONOMIC VALUE DISTRIBUTED TO STAKEHOLDERS’ CATEGORIES IN PERCENTAGE IN 2021 Total economic value distributed by the Group 675,906 million € 62,74% Suppliers 31,31% Personnel 4,65% Public Administration 1,28% Lenders 0,03% Donations 3. Over the course of 2021, shareholders’ remuneration was covered by a withdrawal from the extraordinary reserve, thus not affecting the distribution of the economic value generated in 2021. Specifically, during 2021 dividends amounting to 11.2 million € were distributed to shareholders through the use of the extraordinary reserve.

revenues are reported in both segments and include a detailed breakdown of high-value solutions and other containment and delivery solutions. The figure shows the strong double-digit growth in all business sectors of the Group operational activities, confirming the commitment and efforts of all Group employees. The high-value solutions are complex products, processes and services for which the Group owns the intellectual property rights and has a strong proprietary know-how. 3.2 Key financial results During 2021, the Group continued working actively to support pharma companies in the fight against the Covid-19 pandemic. As a result, it experienced a growth in demand for its high-value solutions as well as other containment and delivery solutions, making 2021 a remarkable year for Stevanato Group with an increase in total revenue of more than 180 million € compared to 2020. The Group demonstrated strong resilience and effective capacity-building in response to the demand, overcoming the difficult challenges of the global situation. Overall, 2021’s increase in revenue, together with the investments in production capacity, provided a solid foundation for further growth in 2022 in both of Stevanato’s business segments, as analyzed below. Stevanato Group’s business segment revenue can be divided into Biophar-maceutical and Diagnostic Solutions and Engineering. In the table below,
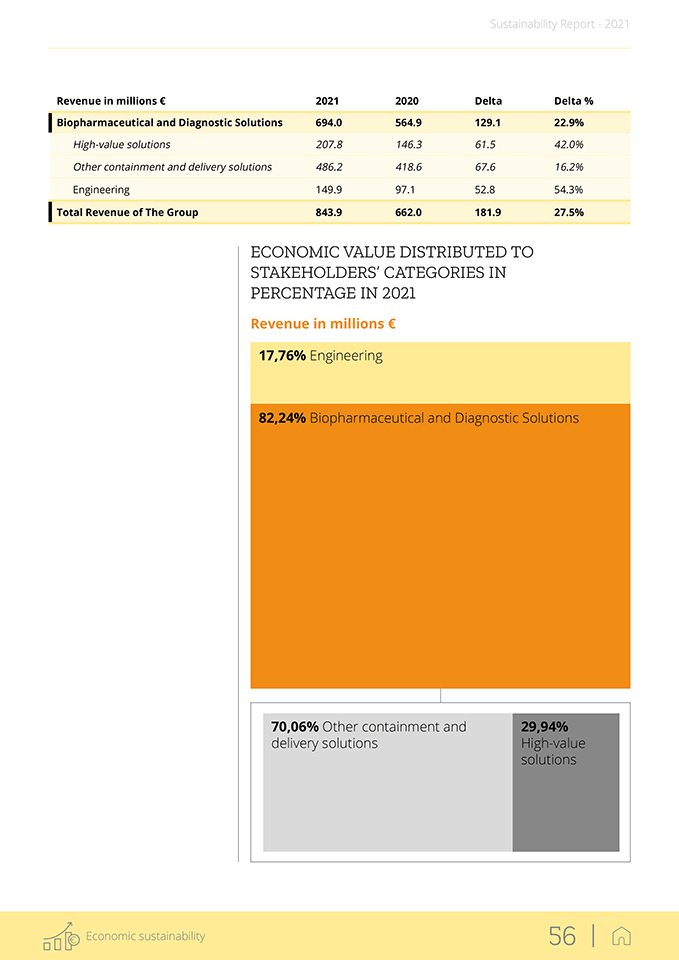
Revenue in millions € 2021 2020 Delta Delta % Biopharmaceutical and Diagnostic Solutions 694.0 564.9 129.1 22.9% High-value solutions 207.8 146.3 61.5 42.0% Other containment and delivery solutions 486.2 418.6 67.6 16.2% Engineering 149.9 97.1 52.8 54.3% Total Revenue of The Group 843.9 662.0 181.9 27.5% ECONOMIC VALUE DISTRIBUTED TO STAKEHOLDERS’ CATEGORIES IN PERCENTAGE IN 2021 Revenue in millions € 17,76% Engineering 82,24% Biopharmaceutical and Diagnostic Solutions 70,06% Other containment and 29,94% delivery solutions High-value solutions
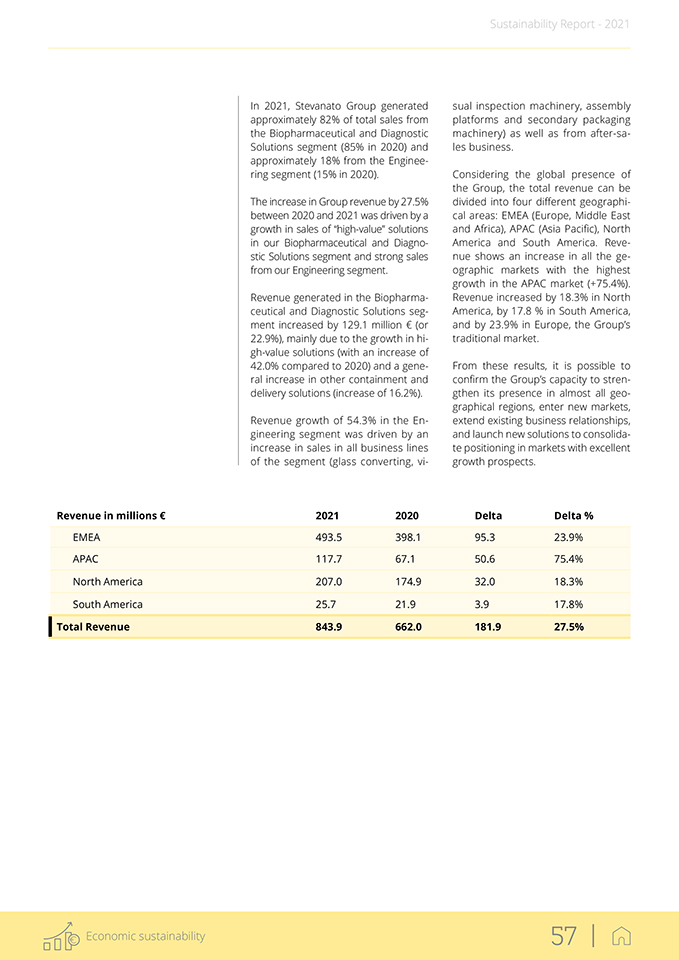
In 2021, Stevanato Group generated approximately 82% of total sales from the Biopharmaceutical and Diagnostic Solutions segment (85% in 2020) and approximately 18% from the Engineering segment (15% in 2020). The increase in Group revenue by 27.5% between 2020 and 2021 was driven by a growth in sales of “high-value” solutions in our Biopharmaceutical and Diagnostic Solutions segment and strong sales from our Engineering segment. Revenue generated in the Biopharma-ceutical and Diagnostic Solutions segment increased by 129.1 million € (or 22.9%), mainly due to the growth in high-value solutions (with an increase of 42.0% compared to 2020) and a general increase in other containment and delivery solutions (increase of 16.2%). Revenue growth of 54.3% in the Engineering segment was driven by an increase in sales in all business lines of the segment (glass converting, vi- sual inspection machinery, assembly platforms and secondary packaging machinery) as well as from after-sales business. Considering the global presence of the Group, the total revenue can be divided into four different geographical areas: EMEA (Europe, Middle East and Africa), APAC (Asia Pacific), North America and South America. Revenue shows an increase in all the geographic markets with the highest growth in the APAC market (+75.4%). Revenue increased by 18.3% in North America, by 17.8 % in South America, and by 23.9% in Europe, the Group’s traditional market. From these results, it is possible to confirm the Group’s capacity to strengthen its presence in almost all geographical regions, enter new markets, extend existing business relationships, and launch new solutions to consolidate positioning in markets with excellent growth prospects. Revenue in millions € 2021 2020 Delta Delta % EMEA 493.5 398.1 95.3 23.9% APAC 117.7 67.1 50.6 75.4% North America 207.0 174.9 32.0 18.3% South America 25.7 21.9 3.9 17.8% Total Revenue 843.9 662.0 181.9 27.5%
REVENUE BY GEOGRAPHICAL LOCATION (MILLION €) 2020 2021 600 493,5 500 398,1 400 300 207 200 174,9 117,7 100 67,1 21,9 25,7 0 EMEA APAC NORTH AMERICA SOUTH AMERICA The results achieved in 2021 are significant and a testimony to the level of excellence offered by Stevanato products and services, as well as the highest standards of professionalism demonstrated by all of the Group’s internal stakeholders.

STEVANATO GROUP HAS RECENTLY CONFIRMED ITS COMMITMENT TO THE FIGHT AGAINST CORONAVIRUS Stevanato Group has been in the vaccine business for decades, serving as a partner for the distribution of diverse vaccines worldwide. In 2020, the global COVID-19 pandemic caused both governments and private organizations to implement numerous measures seeking to contain the spread of the virus. These measures impacted, and are expected to continue impacting, the Group’s business and operations in several ways. Initially, unfavorable short-term impacts of COVID-19 on production and operational capabilities mainly included: (i) a temporary decrease in the sales of certain non-COVID-19 products as a result of traditional healthcare procedures being postponed and the diversion of our production capacity to support the rollout of the COVID 19 vaccine worldwide; (ii) labor absenteeism; (iii) disruptions to production lines; (iv) delays in, and increased costs of, logistics. However, COVID-19 also provided an uplift to the Group’s business with an acceleration of revenue from the sale of syringes and vials for vaccination programs globally. Stevanato Group has been supplying: (i) glass vials and syringes to approximately 90% of currently marketed vaccine programs, according to our estimates based on public information (WHO, EMA, FDA); and (ii) plastic diagnostic consumables for the detection and diagnosis of COVID-19. Going forward, the Group expects demand for syringes, vials and related products and services to remain elevated as the COVID-19 vaccine and treatment programs continue to roll-out globally, and as customers contemplate the transition from multi-dose formats to single-dose formats. In addition, the Group expects continued tailwinds as epidemic preparedness, including the ongoing global COVID-19 vaccine rollout, booster shot distribution and new vaccination programs, remain a priority for governments. Longer-term, there remains uncertainty around the magnitude of the impact of COVID-19 and the demand for our solutions. Many scientists predict that COVID-19 will eventually transition to an endemic state. While timing of this transition is difficult to predict, experts believe that the transition may likely occur over the next twelve to twenty-four months. This may result in a continued need and relatively stable demand for the Group’s products and services related to COVID-19, which would be integrated into the standard vaccine business in the coming years.

Research, development, innovation and product responsibility 4

4.1 Stevanato Group products, technologies and services Stevanato Group is a leading provider of mission-critical containment, delivery and diagnostic solutions for the pharmaceutical, biotechnology and life sciences industries. The figure below provides a breakdown of the Group’s segments, as well as the business lines included within each segment. Stevanato’s two main business segments (Biopharmaceuti-cal and Diagnostic, and Engineering), combined with its global presence, allow it to sell products and provide services in over 70 countries worldwide mainly through business to business marketing channels and selected distributors.
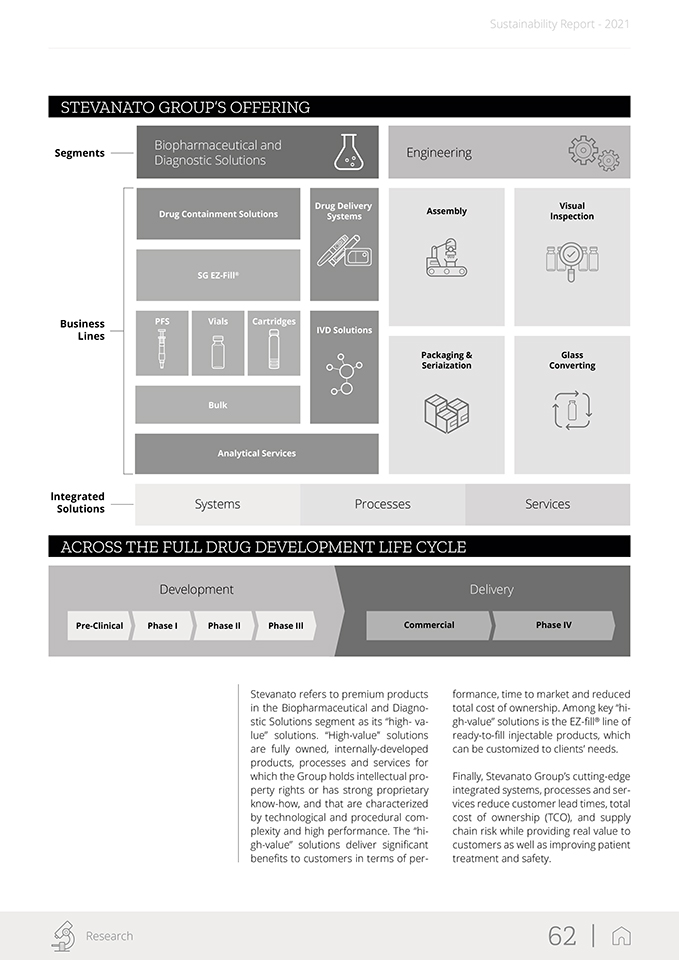
STEVANATO GROUP’S OFFERING Biopharmaceutical and Segments Engineering Diagnostic Solutions Drug Delivery Visual Drug Containment Solutions Assembly Systems lnspection SG EZ-Fill® Business PFS Vials Cartridges IVD Solutions Lines Packaging & Glass Seriaization Converting Bulk Analytical Services lntegrated Solutions Systems Processes Services ACROSS THE FULL DRUG DEVELOPMENT LIFE CYCLE Development Delivery Pre-Clinical Phase I Phase Il Phase IIl Commercial Phase IV Stevanato refers to premium products in the Biopharmaceutical and Diagnostic Solutions segment as its “high- value” solutions. “High-value” solutions are fully owned, internally-developed products, processes and services for which the Group holds intellectual property rights or has strong proprietary know-how, and that are characterized by technological and procedural complexity and high performance. The “high-value” solutions deliver significant benefits to customers in terms of per- formance, time to market and reduced total cost of ownership. Among key “high-value” solutions is the EZ-fill® line of ready-to-fill injectable products, which can be customized to clients’ needs. Finally, Stevanato Group’s cutting-edge integrated systems, processes and services reduce customer lead times, total cost of ownership (TCO), and supply chain risk while providing real value to customers as well as improving patient treatment and safety.

Reduced time Reduced supply Reduced total Consistent to market chain risk cost of ownership quality Unique combination of integrated capabilities to address customer needs across the pharmaceutical value chain Drug containment Drug Delivery Technologies & Analytical Services solutions Systems Manufacturing & Regulatory Equipment Support (incl. TECs) Highly Customized Proprietary Devices and and Pre-Sterilized CMO/CDMO Business Sub & Final Assembly, Analytical Testing & Containment Solutions Automation & Packaging Regulatory Support (SG EZ-fill®) through Solutions, Visual Ompi brand Inspection, Glass Converting Systems Processes Services Research

4.1.1 BIOPHARMACEUTICAL AND DIAGNOSTIC SOLUTIONS Stevanato Group relies on more than 70 years of experience in the production of advanced glass containment solutions for the pharmaceutical and healthcare industries. The Group has evolved from a glassware producer to a full-solution provider, offering integrated capabilities and services along the pharma supply chain. Drug containment solutions SG has a vast range of commercially available drug containment solutions geared towards specific biopharmaceu-tical requirements. Today, the Group is the market leader in manufacturing both pre-sterilized glass vials and glass cartridges for insulin pen injectors, and the world’s second-largest manufacturer of pre-fillable syringes. VALUE CHAIN: ROLES & RESPONSIBILITIES Traditional and Bulk Contain- Container Washing Sterilization Filling ment Manufacturing Solutions DRUG CONTAINMENT PHARMA COMPANY INDUSTRY Containers Container Washing Sterilization Filling Manufacturing DRUG CONTAINMENT INDUSTRY PHARMA COMPANY Ready-to-Use Vials, Cartridges, Syringes Cost Savings: Simplified Reduction of Reduction of costs and Lower Validation Upstream issues associated with CapEx and Regulatory Operations lower quality solutions With traditional or bulk containment solutions, Pharma companies bear the burden of washing, sterilizing and filling containers before distributing Key Benefits REDUCE TCO i.e., logistics, drug product waste, storage and personnel cost INCREASE FLEXIBILITY INCREASE QUALITY REDUCE TIME TO MARKET REDUCE COMPLEXITY

Sustainability Report—2021 When it comes to ready-to-fill products, no solution matches Stevanato Group’s EZ-fill®. It’s a fully integrated, pre-sterilized containment platform for aseptic manufacturing, whose proven advantages have turned its processing technology into an industry standard for other players in the market, reducing complexity thanks to its great processability and pre-verified systems. Available in both nested and tray configurations, EZ-fill® solutions meet the flexibility requirements necessary to accommodate small or large batch production. Acting as a pre-sterilized customizable solution, it helps pharma companies maximize their efficiency by reducing total costs of ownership and time to market. Finally, by minimizing glass-to-glass contact, it improves the integrity of the drug product and ensures patients’ safety. In the following image, the added value of SG EZ-fill® for customers is illustrated. Thanks to its extensive knowledge of containment solutions, Stevanato Group can work together with biophar-ma companies and device manufacturers in the selection of the most appropriate packaging components, providing fully-integrated systems that enhance drug quality, safety and efficacy, as well as operational efficiency and patients’ health. The Group’s portfolio is highly diversified and includes Pre-Fillable Syringes, Vials, Cartridges and Ampoules. Within this portfolio, Stevanato Group provides customers with four main performance levels: FINA®—LDP – NEXA® – ALBA®—offering an array of quality and performance solutions. Below, a summary overview of the four main performance levels: FINA® Is a drug containment solution with a robust process validated for high volume and characterized by high-quality consistency. It is the customizable solution for injectables. LDP (low delamination propensity) vials are innovative borosilicate glass vials that significantly reduce the risk of corrosion and delamination. NEXA® is the next level of quality, specifically designed for high-value sensitive injectables. It is characterized by superior cosmetic quality and improved mechanical resistance thanks to its proprietary highly-automated process and application in No Metal To Glass (NMTG) and No Glass To Glass (NGTG) critical unit operations. ALBA® containers are the breakthrough solution for biologics. They are internally treated with an innovative coating and offer unparalleled performance in reducing ultralow content a) silicone oil droplets, b) any potential product-related substance such as an extractable compound.
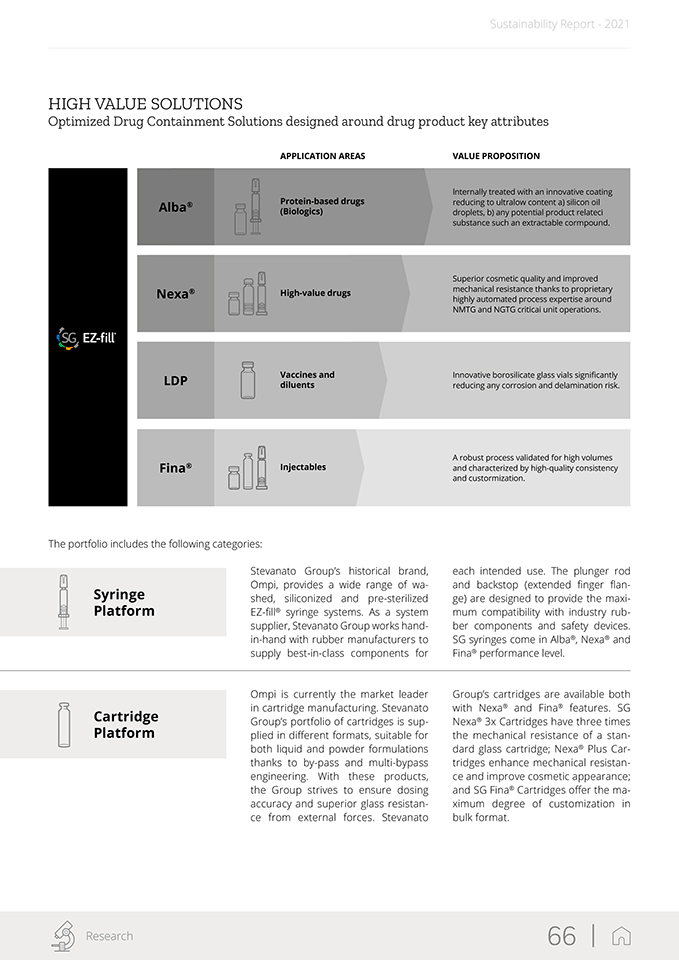
HIGH VALUE SOLUTIONS Optimized Drug Containment Solutions designed around drug product key attributes APPLICATION AREAS VALUE PROPOSITION Protein-based drugs lnternally treated with an innovative coating Alba® (Biologics) reducing to ultralow content a) silicon oil droplets, b) any potential product relateci substance such an extractable cormpound. Superior cosmetic quality and improved Nexa® High-value drugs mechanical resistance thanks to proprietary highly automated process expertise around NMTG and NGTG criticai unit operations. LDP Vaccines and Innovative borosilicate glass vials significantly diluents reducing any corrosion and delamination risk. ® Injectables A robust process validated for high volumes Fina and characterized by high-quality consistency and custormization. The portfolio includes the following categories: Syringe Platform Stevanato Group’s historical brand, Ompi, provides a wide range of washed, siliconized and pre-sterilized EZ-fill® syringe systems. As a system supplier, Stevanato Group works hand-in-hand with rubber manufacturers to supply best-in-class components for each intended use. The plunger rod and backstop (extended finger flange) are designed to provide the maximum compatibility with industry rubber components and safety devices. SG syringes come in Alba®, Nexa® and Fina® performance level. Cartridge Platform Ompi is currently the market leader in cartridge manufacturing. Stevanato Group’s portfolio of cartridges is supplied in different formats, suitable for both liquid and powder formulations thanks to by-pass and multi-bypass engineering. With these products, the Group strives to ensure dosing accuracy and superior glass resistance from external forces. Stevanato Group’s cartridges are available both with Nexa® and Fina® features. SG Nexa® 3x Cartridges have three times the mechanical resistance of a standard glass cartridge; Nexa® Plus Cartridges enhance mechanical resistance and improve cosmetic appearance; and SG Fina® Cartridges offer the maximum degree of customization in bulk format.

Vial Platform Stevanato Group produces glass vials in different sizes and capacities, in bulk or ready-to-fill configuration. With full control over geometry and surface chemistry, the vials can be tailored to meet the different needs of biopharmaceutical customers. The Group’s portfolio also includes special vial containment solutions such as micro-vials for respiratory and su- blingual applications. The performance levels inside this category are: SG Nexa®, which provides the highest level of quality, safety and technology even for the most demanding drugs and low delamination propensity vials (LDP) for aggressive compounds; and SG Fina®, which is highly customizable in terms of geometry, dimension, and tolerances. Ampoules To meet the needs of different pharmaceutical applications, Stevanato Group produces a variety of ampoules compliant with ISO 9187. The Group provides high-quality glass containers for pharmaceutical applications with a broad range of geometries and breakage systems. The production methods and logistical processes for ampoules are compliant with the strict terms and conditions of pharmaceutical and cosmetic markets to ensure maximum adherence to the client’s quality processes. Drug Delivery Systems Over the past few years, Stevanato Group has strategically expanded its drug delivery systems team and broadened its offering to include capabilities and services as an integrated solutions provider. The company is now well-positioned to help its pharmaceutical partners launch drug products to market in a delivery device that suits the needs of patients. Stevanato Group focuses on two main areas with regard to drug delivery systems: Contract Manufacturing and Proprietary and Licensed devices. Through Contract Manufacturing, Stevanato Group provides customers with scalable manufacturing solutions for their drug delivery system programs. Customers can rely on: A complete CDMO or CMO offer, covering different stages of device development, up to the final product; High-quality production standards, thanks to an ISO 13485 certified Quality Management System and FDA inspected facilities; A worldwide presence, with manufacturing operations in Europe and the US and a global purchasing network. Stevanato Group’s Proprietary and Licensed devices include pen injectors, auto-injectors, wearables, and inhalers, as described in the table below.

Pen injectors Alina® is a variable- and fixed-dose disposable pen injector, based on Axis-D technology, exclusively licensed from pen injector device expert, Haselmeier. It is suitable for a broad range of therapeutic areas including diabetes & obesity. Auto-injectors Together with a trusted device partner, Stevanato Group is developing an innovative auto-injector solution that offers flexibility and speed-to-market for customers. The intuitive design is suitable for a broad range of therapeutic areas. Wearables Stevanato Group is developing a semi-reusable cartridge-based wearable device composed of two systems: a disposable pod and a reusable cradle. It is optimized for the administration of complex drug regimens from mid-high volume cartridges. Inhalers ICOcap® is an easy-to-use capsule-based dry powder inhaler licensed from inhalation specialist, Iconovo. It incorporates improved handling with equivalent performance-to-market products that are used to treat asthma and COPD. IVD Solutions At Balda, Stevanato Group experts are specialists in multi-component injection molding and automated system assembly. As a one-stop solution provider and manufacturer, the Group can cover all parts of the process, from product development to delivery of the final product, packaged and sterilized as needed. With Balda’s over 110 years of company history, Stevanato Group has gained unique knowledge of large-scale manufacturing of plastic components as well as complex assembly. As a leading producer, the Group combines the highest quality standards and economies of scale for large volumes and high product complexity. Stevanato Group plastic solutions are available in all three market areas: pharmaceutical, with intelligent plastic products and solutions based on extensive product development capabilities; medical, with the development and manufacture of medical plastic solutions with high functionality; and diagnostic, as point-of-care consumables and modern handheld devices. Analytical services As a branch of Research and Development, the analytical testing facilities focus on investigating the physical-chemical properties of primary packaging materials and components, and studying the interactions between Drug Containment Solutions and drugs. Stevanato Group relies on a multi-disciplinary team of highly-skilled professionals including scientists, engineers, pharmaceutical chemists and biotechnologists. Their knowledge and experience cover a range of specialized areas including Drug Containment Solutions and drug-delivery devices.

These services can be divided in: • Primary Container Compatibility and Functionality with Drug Product A series of characterization techniques that aims to fully understand how the primary packaging behaves in the presence of the drug product and/or under specific applications or conditions with a deterministic approach. • Container Closure Integrity Methodologies and techniques that challenge the sealing integri- ty of a container closure system to ensure the correct compatibility of materials and storage conditions. • Medical Devices/Combination Products Extensive testing to ensure the robustness of product functionality. • Developmental (non-GMP) Fill & Finish Service Small, flexible Fill & Finish equipment to perform preliminary work on drug product, process, or container optimization.

4.1.2 ENGINEERING Stevanato Group can count on a vast portfolio of technologies and manufacturing equipment to best serve its customers. First, Stevanato Group is specialized in glass converting, producing fully-automated, high-speed, precision glass tube converting lines through its brand, Spami. The Group can provide accurate processing of ampoules, vials, cartridges and syringes throughout the development process, and rigorous product inspection via state-of-the-art camera control systems. The manufacturing lines are certified and validated to process high quality type 1 glass all over the world. In addition, the Company provides an accurate after-sales service and a 360° support and consultancy, designing customers’ plant layouts for the production of bulk and ready-to-use pharmaceutical primary packaging. Secondly, Stevanato Group provides flexible inspection solutions at any stage of the product journey, from lab development to high-volume production. Through Optrel and InnoScan brands, the Group provides inspection solutions for ampoules, vials, cartridges, syringes or bottles filled with clear or opaque liquids, emulsions, viscous gels, lyophilized products and other difficult-to-inspect solutions. The inspection machines can be divided into manual, semi-automatic and automatic equipment. Every feature is specific to the customer’s needs, with optimal synchronization of high-performance CMOS cameras, illumination and con- tainer rotation that enable highly efficient, multi-inspection of each primary container. Regarding this aspect, the Group has launched Stevanato Group Vision AI – a secure, cloud-based platform featuring Deep Learning models that increase the detection rate while minimizing the false rejection rate (see Chapter 4.2). Finally, the Group is equipped to offer device assembly and packaging equipment. Stevanato Group designs and manufactures flexible stand-alone or turnkey integrated systems to address and support customers’ requirements from the early stage up to commercialization. It delivers both precision assembly of a wide variety of medical devices, including pen injectors, auto-injectors, nasal sprays, inhalers, wearables, cartooning machines, packaging machinery and palletizing modules, and integrates equipment compliant with serialization and tamper-evident requirements. SG’s engineering leadership Master Plan aims to structure and guide the operational transformation of the engineering division of SG, defining a detailed road map to achieve success and consistent execution. The approach includes tangible milestones that will help track the business’ success. In addition, the approach is based on the competence, experience and preparation of the entire engineering team which is committed to exceeding the standards.

4.2 R&D and Innovation Research and development investment is a fundamental component of Stevanato Group’s growth and continued success. The company invested approximately 29.6 million € in 2021 (3.5% of revenue) compared to 12.2 million €. The research and development team comprises more than 140 highly skilled and specialized employees operating in Italy, Germany and the United States. R&D also represents a dimension of Stevanato Group’s vision, allowing it to evolve from a packaging component and automated equipment supplier to a leading provider of mission-critical drug containment, delivery and diagnostic solutions for the pharmaceutical, biotechnology and life sciences industries. The Group believes that customers trust the company because of its technical expertise and ability to design the best possible process to meet their requirements and the specifications necessary to effectively contain and deliver their drugs. Thanks to numerous investments in internal engineering capabilities, Stevanato Group is the owner of the most critical processes behind its manufactured products and is able to respond faster to customers’ needs for new or customized products. SG’s engineering capabilities also allow it to scale up production rapidly when required, thereby reducing the lead time for the commercialization of drugs. Investment in innovative products and technologies will continue to be a fundamental component of growth and success going forward, allowing the Group to capture incremental pipeline opportunities and drive attractive growth. Being involved in the customer’s drug development process at an early stage allow Stevanato Group’s R&D department to deliver science-based innovation through various services, including: • Clinical, laboratory and registration services with its laboratory and Technology Excellence Center, such as the one opened in Boston, Massa-chusetts in 2020. • High-quality product development, including in-depth risk analysis, to ensure a standardized project execution process. • Definition of new proof-of-concept with process, automation and robotics in the device assembly, testing and inspection phases. • Advanced technical customer support benefiting from experts in the field of materials science (such as glass, plastics, polymers, coating, rubbers and proteins), chemical science, mechanical engineering and medical devices. • Generation of technical and regulatory documentation fully compliant with global regulations such as those of the US Food and Drug Administration, China Food and Drug Administration and European Medicine Agency.

Drug Containment Solutions Developing solutions to maintain the stability, potency and purity of biopharma customers’ products prior to administration Drug Delivery Systems Focusing on DDS patient-centricity, sustainability and digitalization Process Excellence & Digitalization Continuously innovating the Group manufacturing processes in order to deliver superior quality, reducing waste and risk of drug shortages From a company perspective, the R&D department is divided into two divisions in line with Stevanato’s business segments: Biopharmaceuti-cal and Diagnostic Solutions, and Engineering. The two R&D divisions cooperate in perfect synergy in terms of maximizing value creation and accomplishing cross-functional projects. The mutual strategy of Stevanato Group’s R&D department is based on three fundamental pillars that focus and align the R&D team with the Company’s business growth: These three pillars are the guidelines of the Group’s R&D divisions: each R&D project is designed to contribute and support one or more of these pillars. 4.2.1 R&D FOR DRUG CONTAINMENT SOLUTIONS (DCS) The R&D team of the Biopharmaceuti-cal and Diagnostic Solutions segment is responsible for the development of many important Stevanato Group products and features, including the Drug Containment Solutions (DCS) portfolio. Regarding the first pillar, the DCS team is continuously developing syringes, vials and cartridges with the lowest particle generation, reduced or even no extractable release, and a metal-free option. In addition, the team is responsible for the improvement of deep freeze/dead volume properties of Stevanato solutions to refine the Vaccine and Gene Therapies application. To deliver superior quality Process Excellence & Digitalization, the Drug Containment So- lutions team is working on different assignments, among which, the development of a unique identification and serialization of individual containers, the integration of Artificial Intelligence to improve visual inspection, and the use of virtual prototypes that accelerate development of inspection and assembly. Finally, the DCS team projects also contribute to the development of Drug Delivery Systems patient-centricity, sustainability and digitalization, especially by providing the best primary packaging fully compatible with the devices under development. The main innovations included in the Drug containment solutions portfolio are: ALBA® Platform ALBA® is a sterile glass container platform including vials, and syringes with equivalent chemical and mechanical characteristics. All the ALBA® products have an internal lubricant layer which is cross-linked with the surface of the glass. The advantage given by this innovative platform is in limiting the quantity of silicone that is then released by the primary container, thereby improving both the quality and safety of the final product. Furthermore, the superior stability of the lubricant layer ensures no failures with self-administration injection related to auto injector applications, allowing for improved compliance with therapies. ALBA® involves several different internal and external companies with competences that range from design and testing to manufacturing. In 2021, its main activities were focused on assessing alternative grades of silicone as coating materials, thus obtaining an even more improved and reproducible product.

Integrated Safety System (ISS) The Integrated Safety System is an advanced, fully passive shield that prevents needle-stick injuries. ISS Integrated Safety System aims to offer a pre-assembled system ensuring passive needle protection after the injection of medicinal products for use in both hospital and household environments. It benefits not only the end-user thanks to its simple user experience and its intuitive application, but also the pharma-company with a reduced Total Cost of Ownership (TCO). It is an active project that involves more than twenty different internal and external companies which represent the full spectrum of expertise, from Safety System design development to industrial production. In 2021, the first commercial product based on our ISS system was approved by Regulatory Authorities and launched on the market. Primary Container Traceability This project permits the traceability of the container in every part of the manufacturing process. Every container can be marked with a unique serial number identifier and traced with a 2D barcode-reading machine. Primary Container Traceability enables the manufacturers to improve the drug life cycle by facilitating the tracking of potential issues within each process stage, preventing product mix-ups, permitting segregation in the event of a process issue, improving patient safety and maintaining ownership of the product’s identity even if the packaging/label has been damaged. Feasibility studies of the concept have been completed, allowing next steps to be taken in project development. In addition, Stevanato Group began looking into sustainability measures as part of its corporate Circular Economy innovation program. In particular, projects were mobilized to identify alternative materials and processes that would provide more sustainable sterilization techniques while maintaining the overall performance of materials post-sterilization cycle. The possibility of identifying the best material to be used across different products and sterilization conditions would give the Group a significant advantage in terms of simplifying and improving its long-term supply chain. SG is also actively collaborating with the scientific community and universities to advance its scientific insight into the Group’s current and prospective product line, as well as to provide its customers with the latest know-how on specific products. In certain research areas, including chemical-physical and morphological characterization of glass surfaces and drug interactions, we cooperate with universities such as Ca’ Fo-scari University (Venice, Italy), Federico II University (Naples, Italy), the National University of Ireland Mynooth (Ireland), and the University of Trento (Italy). 4.2.2 R&D ON DRUG DELIVERY SYSTEMS (DDS) In addition to the activities related to Drug Containment Solutions, a fundamental responsibility of Biopharma-ceutical and Diagnostic Solutions R&D is the development and expansion of Stevanato Group’s Drug Delivery Sy- stems (DDS) portfolio. This includes developing innovative delivery solutions that are focused on usability, safety, performance and manufacturability. The main projects on which the department is working are the following:

Alina® is a disposable, multi-dose pen injector platform for subcutaneous administration of injectable therapy. The platform is available in variable and fixed-dose versions compatible with established therapeutic regimens as well as innovative drug therapies related to diabetes care. The project involves multiple SG sites and functions and an international design and development team. An Auto-injector Platform is critical to the growth strategy of Stevanato Group’s integrated offering. This is supported by the ongoing market demand for auto-injector solutions. The R&D team continues to assess various auto-injector technologies, including both non-emergency use and emergency-use devices, while considering its environmental footprint as a key determinant of product suitability. Stevanato Group’s Wearable Product is designed for subcutaneous delivery of non-opioid anesthetics. The device enables controlled self-administration of therapy while reducing unnecessary hospital stays. The re-usable cradle component extends the lifespan of the product to multiple-u-ses and reduces product waste for a more sustainable device solution. This is the first of many steps in SG’s corporate Circular Economy innovation program. The overall concept of this wearable product can be extended to a platform capable of large volume delivery from different cartridges. ICOcap® is a capsule-based dry-powder inhaler for the treatment of asthma, chronic obstructive pulmonary disease (COPD) and other respiratory-related diseases. It has similar performance to established inhalers on the market while offering improved handling features. The project involves multiple SG sites and functions and an international design and development team. 4.2.3 R&D ON ENGINEERING To advance R&D in the Engineering segment, the Group has invested in the latest equipment and expanded its lines and services to improve production and best serve its customers in four main product areas: Glass tube Converting, Visual Inspection, Assembly and Packaging. The Engineering department aims to achieve high performance and to understand internal and external expectations in order to clearly guide product development in the following directions: • High performance: line and equipment capable of achieving superior operating performance in the long term (OEE, Overall Equipment Effectiveness) whilst being cost effective and reducing the TCO (Total Cost of Ownership). • Quality and reliability: completion of the portfolio range based on modular architecture with the main target being a stable platform able to minimize planned, and avoid unplanned, downtime.

• Smart and connected equipment and lines: development of smart solutions by building machine intelligence into equipment via features like prediction, aided guidance and self-adjustment. Machines and lines are connected in a cyber-secure way to the cloud where big data technology can generate valuable information through advanced data analytics. The Deep Learning models embedded in the visual inspection solutions provide customers with an efficient system to assist them in significantly reducing the FRR (False Rejection Rate) and increasing the DR (Detection Rate). The Engineering quality control systems are designed to ensure that the manufacturing processes, including those of pharma customers and contracted manufacturing companies, comply with Generic Good Practice (GxP) standards based on the Good Automated Manufacturing Practice (GAMP) guidelines issued by the International Society for Pharmaceutical Engineering (ISPE). Each individual piece of machinery/equipment is developed and manufactured as a project and ad-hoc project management tools are utilized to manage every stage and minimize risk. Similarly to the Biopharmaceutical and Diagnostic Solutions R&D division, the Engineering division contributes to the three R&D pillars, in particular, to Process Excellence & Digitalization. Below are the main projects under Engineering R&D essential to promoting innovation and increasing Stevanato Group’s competitiveness and market share. Smart Factories – the Group has designed its production plants to be highly interconnected with the objective of: • Performance monitoring thanks to an automatic real-time data acquisition system that continuously controls the performance of equipment and lines. The system is equipped with a simple and intuitive user interface on fixed and portable devices, and is fully integrated with the Human-Machine Interface (HMI) equipment at all levels, from shop floor to corporate offices. • Improvement in solving capabilities with equipment that, in case of a problem, offers step-by-step guidance via accelerated Root Cause Analysis (RCA). After the analysis, the equipment guides the operator through the problem resolution process and stores all the pertinent

Sustainability Report—2021 information in a Big Data repository in order to improve the next RCA. • Improvement in predicting capabilities through the constant analysis and evaluation of data and operating parameters in machines and lines, helping to anticipate future downtime. • Automatic adjustment via equipment capable of setting optimal ranges for glass formation without the need of operator expertise. Also maintains long-term high-quality production while minimizing energy consumption. • Video Remote Assistance with real-time video support from a remote technician able to assist the on-site operator dealing with a machine operation problem. • Artificial Intelligence applied to Visual Inspection – Stevanato Group has started using artificial intelligence (AI) in visual inspection to address the problem of false rejects. AI enables the system to correlate the actual image of the product under inspection with a vast library of similar images of accepted and rejected products, thereby identifying the likelihood of it belonging to one of the categories. The phar-ma industry has been actively studying this technology over the past two years and Stevanato Group is among the forerunners, with the expectation of increasing its market share in the future. • With standard visual technology, operators use classical parameters of measurement, using their own experience and taking samples from production. “False rejects” are the inevitable trade-off of having access to a limited set of samples. Sometimes good products are rejected out of extreme caution. Research 76 |

Digital Twin the device is a virtual replica of a machine or line with examples of application on both Assembly and Visual Inspection. The customer can review its performance and interact virtually with the equipment before going into production. By experiencing the machine in advance, the customer can anticipate possible problems and reduce the production time needed once a decision is made. The digital twin can also be used both as a promotional tool and as a substitute for a possible test phase. Vision Robot Unit the Visual Robot Unit represents a new product within the product portfolio of Stevanato Group’s Pharma Vision area. It consists of a new type of automatized production machine that allows for flexibility during manual inspection along with the replicability and stability given by automatic equipment. It aims to address two main market needs: the demand for improved inspection performance and an extremely flexible unit to satisfy low-volume markets. The objective is to allow the Group to increase its position within emerging markets determined by low-production output per minute and high value of the product to be inspected. New SW and HW Platform for Inspection Equipment4 SG heavily invested in a new SW platform in 2021 aiming to improve performance and increase customer satisfaction. The platform merges know-how from both InnoScan and Optrel brands, whose combined resources have advanced overall efficiency and reduced timelines for the final customer. Moreover, the new SW platform leverages a series of new inspection tools which can improve the final DR (Detection Rate) and FRR (False Rejection Rate). Lines development improvements SG is developing new lines with the purpose of reducing capital expenditures and industrial costs, increasing productivity and improving in-line quality control. Such projects also involve syringe systems production. 4. CVT, CVT Sy and CVT Core Research 77 |

Sustainability Report—2021 Assembly | CollectQX data collection tool: CollectQX was developed in cooperation with a pharmaceutical company to create the best solution for data analysis, easy interface and future implementation of Artificial Intelligence (AI). The focus was to overcome boundaries of known data collection tools and create complete data transparency by connecting all the necessary in- formation. The unique tool is useful for the Group during running-in as well as for customer operations, maintenance and data analytics (please see the image below to better understand the project scope). The Engineering R&D projects noted above involved more than 30 highly-skilled employees. Batch Recipe Quality checks/-data Machine ID Product ID Workposition ID All Parameter Serialization ID Timestamp(s) All connected to each single Datapoint 4.2.4 ANALYTICAL SERVICES Another important branch in R&D for Ste-vanato Group is Lab Analytics. Its goal is to investigate and analyze chemical-physical phenomena and study the interactions between containers and drugs. In 2012, SG Lab started out as a laboratory staffed by a small group of young scientists, whose ranks soon began to grow. Today, SG Lab has a staff of more than 15 people including researchers, scientists, engineers, laboratory technicians and people in charge of ISO 17025 and, starting in 2021, ISO 13485 quality-systems management. The team can rely on members trained in the fields of chemistry, engineering, physics and pharmaceutical sciences who are able to examine and define each aspect of a drug product. Their knowledge ranges from add-on components to the performance of glass primary containers and how they interact with the drug. They also have a series of skills acquired and developed in the study and analysis of drug-container closure system and delivery devices. Staff training and development has always been a fundamental part of Stevanato Group’s agenda. The Group continues to invest in modern analytical instruments and in the acquisition of new skills, both internally and through external collaboration with universities and analytical excellence centers. Its personnel can rely on a solid knowledge base and a technical-scientific approach, enabling it to tackle future challenges with confidence. Research 78

Sustainability Report—2021 US TEC AT THE HEART OF THE BIOTECH COMMUNITY, BRINGING VALUE TO STEVANATO GROUP’S LABORATORY SERVICES To respond to the growing demand in the US biologics market for integrated analytical offerings and project management services, in September 2020 Stevanato Group established a Boston-based laboratory and Technology Excellence Center (TEC). US TEC can be considered an additional R&D and investigational testing facility. Its services can help determine new ways of improving product quality or identify the cause of any current quality issues. The laboratory is modelled after the SG Laboratories (with the same QMS system) and provides a full-service approach to support biopharma companies along their drug development journey, from the early phase to commercialization and lifecycle management. Leveraging the Group’s extensive knowledge in materials science of glass, plastics and rubber, US TEC supports one of the most critical decisions for biopharma industry players: how to select the optimum glass container. Making the right choice during the early-stage of product development ensures two-fold compatibility: Drug and Container Closure System as well as Container Closure System integration into Drug Delivery Systems. Since R&D represents a key driver for Stevanato Group’s growth, the US TEC advises on materials science, chemistry and engineering performance. Located in the cradle of the biotech region, US TEC easily integrates into the product development team and the value chain of local customers. In addition to supporting Stevanato Group’s existing large company partners, US TEC offers an opportunity for new, smaller to mid-sized companies to collaborate with the Group’s wide offering. The long-term objective of US TEC is providing a continuum of representation from container to device management. Research 79 |

Sustainability Report—2021 4.3 Product quality and responsibility As a commitment to the production of high-quality products, Stevanato has adopted a Group Quality Policy which aims to collaborate with its customers throughout the life cycle of their products from concept development to commercialization and post-sales support. The policy reflects SG’s dedication to delivering high quality products, advanced technologies and services that fulfill and anticipate its customers’ needs while ensuring end-user safety. To ensure product quality, it is important to note the following ISO certifications: • ISO 15378 on primary packaging material for medicine products – the certification is effective for Nuova Ompi (Padua and Latina), Medical Glass, Ompi North Ameri-ca, Ompi of China, Ompi do Brasil and Balda Germany. • ISO 13485 medical devices quality management systems – the certification is effective for Nuova Ompi (Padua), Balda Germany and Balda USA, and SG Lab Analytics. • ISO 9001 quality management systems – the certification is effective for Nuova Ompi (Padua and Latina), Medical Glass, Ompi North America, Ompi of China, Ompi do Brasil, Bal-da Germany, Balda USA, Spami and SG Lab Analytics. • ISO 17025 on general requirements of competence of testing and calibration laboratories, effective for SG Lab Analytics. Research 80

Sustainability Report—2021 Everyone in Stevanato Group, from senior management to individual employees, is accountable and strives to ensure the continuous improvement and effectiveness of Stevanato Group Quality Management System and Steva-nato Group Quality Policy. FOCUS ON QUALITY Among all its Drug Containment Solutions plants worldwide, the Group provides the same quality of product. The main advantage of this feature is that it best supports business continuity for SG customers: by producing the exact same product worldwide, with documented corporate audits, the company is able to manage risk mitigation and offer production flexibility. To reach the same level of quality across all SG sites, the Group ensures continuous alignment among its plants through product experts in Operational Excellence and Quality that support each site in case of issues, creating a portfolio of knowledge that is continuously shared among the sites. Regular trainings and discussions within the quality department allow all sites to stay up-to-date in both product quality and quality compliance, and keep abreast of industry trends in order to meet the highest regulatory and quality compliance standards. In general, the impact of product quality and responsibility is directly related to Stevanato Group’s activities. In fact, the primary goal for everyone involved in the production process is linked with Stevanato Group’s mission to create systems that enhance the integrity of parenteral medicines, ensuring maximum quality of the primary packaging. For example, pharmaceutical containers are integral to the pharmaceutical product itself, therefore their quality affects patient safety. In this regard, all employees are accountable for following Stevanato Group Quality Management System in order to maximize the quality and integrity of its products. Drug containment, diagnostic and delivery solutions are often borne out of years of collaboration with customers to develop the optimal manner of containing a drug product and delivering it to the patient community. The customized solutions provided vary depending on the characteristics and chemical composition of the pharmaceutical products, logistics needs (for example, ease of transport and shelf-life), factors such as the patient community to which the drug product is primarily addressed (including, potentially, its geographic location) and specific regulatory requirements. The containment and delivery solution provided is an integral part of the drug product itself and is included as part of the regulatory filings required to approve drug product marketing and commercialization. The quality and dependability of drug containment and delivery solutions is critical to obtaining commercialization and marketing approval from regulatory agencies. As a result, it is often the case that drug product containment and delivery solution cannot be changed without amending the regulatory filings that have been specifically approved by the relevant regulatory agency. During 2021, there were no incidents of non-compliance with regulations and/ or voluntary codes concerning the health and safety impacts of products and services within the reporting period. Research 81

Sustainability Report—2021 SG STEPS PROGRAM: FOCUS Customer Satisfaction Process digitalization & Quality Mindset PQ ET FI PM AM EQS SA Education & Progressive Focused Planned Autonomous Efficiency Safety Training Quality Improvement Maintenance Maintenance Quest System 5S PCS Performance Control System SG Steps Program leads the way to a World Class Operations Management (WCOM) transformation. The program centers on a common operating model for all the various processes at each of the manufacturing locations and involves staff working in operations and supporting functions. SG Steps is a rigorous system built on Stevanato Groups’ business and industrial capabilities to address the demands of pharmaceutical customers with the same quality level worldwide. It is developed and validated according to the best practices in the pharmaceutical and industrial sectors. SG Steps embodies Stevanato Group’s ambition to become the best-in-class in the reference field, adopting automation, digitalization and quality as main drivers. It covers all the high value-added processes, empowering each employee with the ideal degree of choice and autonomy, providing greater accountability and ownership in their work. SG Steps Program has three phases, starting from the identification of safety priorities and the creation of a shared plan for continuous improvement –Foundation Phase – up to the implementation of safety standards and the achievement of behavior-driven safety culture – Advanced Phase. The final Mastery Phase integrates safety with new product and process development. To implement the SG Steps Program, the Group follows two baselines – Performance Control System and the 5S methodology – and seven pillars – Safety, Focus Improvement, Efficiency Quest System, Autonomous Maintenance, Planned Maintenance, Education and Training and Progressive Quality. With focus on the last pillar, Stevana-to Group accompanies its customers through the life cycle of their products, setting up a self-sustaining standardized system that delivers the highest possible quality, anticipating their needs and ensuring end-user safety. Research 82 |

Attention to human capital 5

Sustainability Report—2021 5.1 Stevanato Group’s human resources5 In order to meet the demands of a constantly evolving market and accompany Stevanato Group’s evolution and mission, the HR Department’s main strategic objective is to build the best team possible to sustain the company’s growth. An efficient team is an asset to the organization and can boost performance and success by tapping into their unique strengths. The mutual growth of the Group and the team creates a solid foundation on which the company can thrive. This objective has also been confirmed through the materiality analysis that brought to light the importance of Human capital management and development and Employee wellbeing as material topics according to both the Group’s and stakeholders’ perspectives. In detail, the HR strategy is focused on: • Hosting training courses in order to develop technical, managerial and organizational skills for employees’ continuous improvement. • Promoting talent attraction in the context in which the Group operates. • Advancing inclusion policies, diversity management plans and equal opportunities, including equal pay for equal roles. • Setting up tools for assessing employee performance to help individual employees in their personal growth path. • Implementation of welfare programs to support both the employee’s working and private life. • Preparing initiatives in favor of work flexibility (working hours, smart working, etc.) and encouraging initiatives to support employees’ spending capacity. • Supporting physical and mental health plans for employees. 5. All data reported in this chapter is extracted directly from a computer system (Zucchetti database) and is related to all group entities except for Medirio SA, Ompi of Japan Co., Ltd. Human capital 84
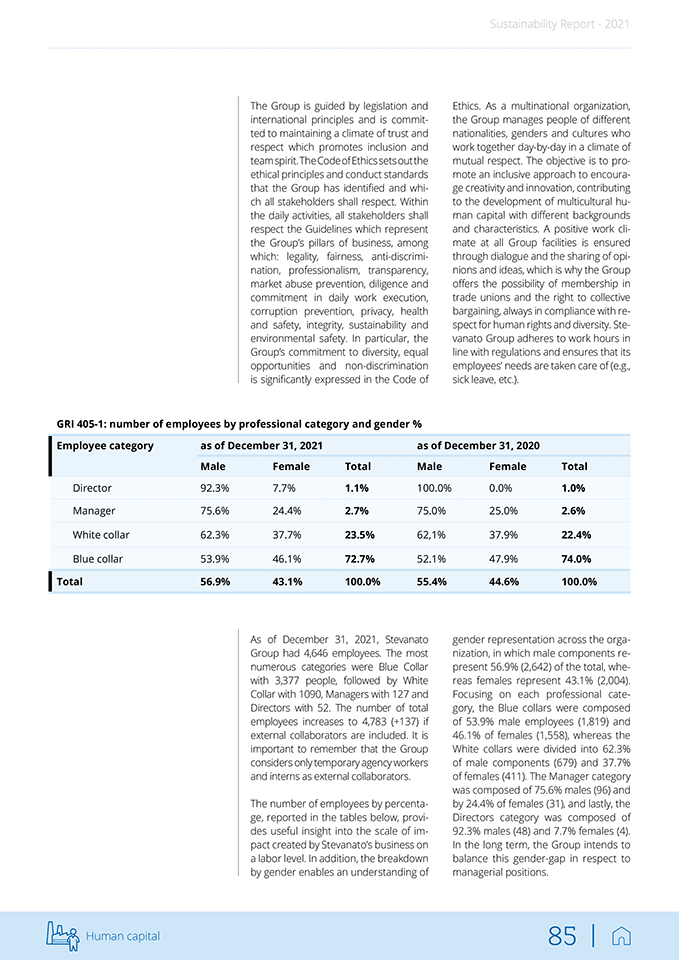
Sustainability Report—2021 The Group is guided by legislation and international principles and is committed to maintaining a climate of trust and respect which promotes inclusion and team spirit. The Code of Ethics sets out the ethical principles and conduct standards that the Group has identified and which all stakeholders shall respect. Within the daily activities, all stakeholders shall respect the Guidelines which represent the Group’s pillars of business, among which: legality, fairness, anti-discrimination, professionalism, transparency, market abuse prevention, diligence and commitment in daily work execution, corruption prevention, privacy, health and safety, integrity, sustainability and environmental safety. In particular, the Group’s commitment to diversity, equal opportunities and non-discrimination is significantly expressed in the Code of Ethics. As a multinational organization, the Group manages people of different nationalities, genders and cultures who work together day-by-day in a climate of mutual respect. The objective is to promote an inclusive approach to encourage creativity and innovation, contributing to the development of multicultural human capital with different backgrounds and characteristics. A positive work climate at all Group facilities is ensured through dialogue and the sharing of opinions and ideas, which is why the Group offers the possibility of membership in trade unions and the right to collective bargaining, always in compliance with respect for human rights and diversity. Ste-vanato Group adheres to work hours in line with regulations and ensures that its employees’ needs are taken care of (e.g., sick leave, etc.). GRI 405-1: number of employees by professional category and gender % Employee category as of December 31, 2021 as of December 31, 2020 Male Female Total Male Female Total Director 92.3% 7.7% 1.1% 100.0% 0.0% 1.0% Manager 75.6% 24.4% 2.7% 75.0% 25.0% 2.6% White collar 62.3% 37.7% 23.5% 62,1% 37.9% 22.4% Blue collar 53.9% 46.1% 72.7% 52.1% 47.9% 74.0% Total 56.9% 43.1% 100.0% 55.4% 44.6% 100.0% As of December 31, 2021, Stevanato Group had 4,646 employees. The most numerous categories were Blue Collar with 3,377 people, followed by White Collar with 1090, Managers with 127 and Directors with 52. The number of total employees increases to 4,783 (+137) if external collaborators are included. It is important to remember that the Group considers only temporary agency workers and interns as external collaborators. The number of employees by percentage, reported in the tables below, provides useful insight into the scale of impact created by Stevanato’s business on a labor level. In addition, the breakdown by gender enables an understanding of gender representation across the organization, in which male components represent 56.9% (2,642) of the total, whereas females represent 43.1% (2,004). Focusing on each professional category, the Blue collars were composed of 53.9% male employees (1,819) and 46.1% of females (1,558), whereas the White collars were divided into 62.3% of male components (679) and 37.7% of females (411). The Manager category was composed of 75.6% males (96) and by 24.4% of females (31), and lastly, the Directors category was composed of 92.3% males (48) and 7.7% females (4). In the long term, the Group intends to balance this gender-gap in respect to managerial positions. Human capital 85
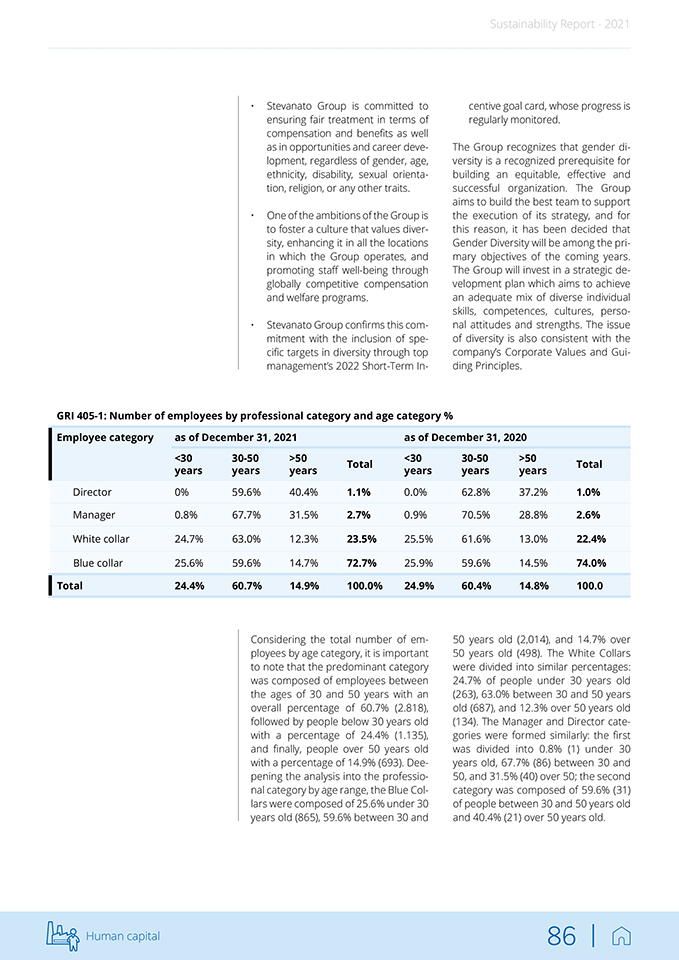
Sustainability Report—2021 • Stevanato Group is committed to ensuring fair treatment in terms of compensation and benefits as well as in opportunities and career development, regardless of gender, age, ethnicity, disability, sexual orientation, religion, or any other traits. • One of the ambitions of the Group is to foster a culture that values diversity, enhancing it in all the locations in which the Group operates, and promoting staff well-being through globally competitive compensation and welfare programs. • Stevanato Group confirms this commitment with the inclusion of specific targets in diversity through top management’s 2022 Short-Term In- centive goal card, whose progress is regularly monitored. The Group recognizes that gender diversity is a recognized prerequisite for building an equitable, effective and successful organization. The Group aims to build the best team to support the execution of its strategy, and for this reason, it has been decided that Gender Diversity will be among the primary objectives of the coming years. The Group will invest in a strategic development plan which aims to achieve an adequate mix of diverse individual skills, competences, cultures, personal attitudes and strengths. The issue of diversity is also consistent with the company’s Corporate Values and Guiding Principles. GRI 405-1: Number of employees by professional category and age category % Employee category as of December 31, 2021 as of December 31, 2020 <30 30-50 >50 <30 30-50 >50 Total Total years years years years years years Director 0% 59.6% 40.4% 1.1% 0.0% 62.8% 37.2% 1.0% Manager 0.8% 67.7% 31.5% 2.7% 0.9% 70.5% 28.8% 2.6% White collar 24.7% 63.0% 12.3% 23.5% 25.5% 61.6% 13.0% 22.4% Blue collar 25.6% 59.6% 14.7% 72.7% 25.9% 59.6% 14.5% 74.0% Total 24.4% 60.7% 14.9% 100.0% 24.9% 60.4% 14.8% 100.0 Considering the total number of employees by age category, it is important to note that the predominant category was composed of employees between the ages of 30 and 50 years with an overall percentage of 60.7% (2.818), followed by people below 30 years old with a percentage of 24.4% (1.135), and finally, people over 50 years old with a percentage of 14.9% (693). Deepening the analysis into the professional category by age range, the Blue Collars were composed of 25.6% under 30 years old (865), 59.6% between 30 and 50 years old (2,014), and 14.7% over 50 years old (498). The White Collars were divided into similar percentages: 24.7% of people under 30 years old (263), 63.0% between 30 and 50 years old (687), and 12.3% over 50 years old (134). The Manager and Director categories were formed similarly: the first was divided into 0.8% (1) under 30 years old, 67.7% (86) between 30 and 50, and 31.5% (40) over 50; the second category was composed of 59.6% (31) of people between 30 and 50 years old and 40.4% (21) over 50 years old. Human capital 86 |
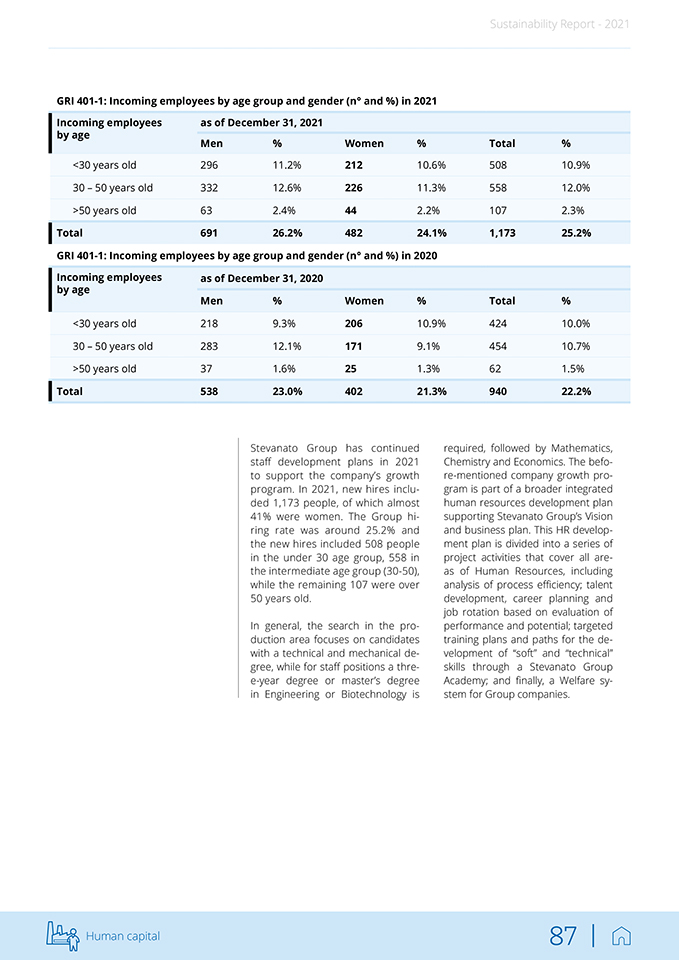
Sustainability Report—2021 GRI 401-1: Incoming employees by age group and gender (n° and %) in 2021 Incoming employees as of December 31, 2021 by age Men % Women % Total % <30 years old 296 11.2% 212 10.6% 508 10.9% 30 – 50 years old 332 12.6% 226 11.3% 558 12.0% >50 years old 63 2.4% 44 2.2% 107 2.3% Total 691 26.2% 482 24.1% 1,173 25.2% GRI 401-1: Incoming employees by age group and gender (n° and %) in 2020 Incoming employees as of December 31, 2020 by age Men % Women % Total % <30 years old 218 9.3% 206 10.9% 424 10.0% 30 – 50 years old 283 12.1% 171 9.1% 454 10.7% >50 years old 37 1.6% 25 1.3% 62 1.5% Total 538 23.0% 402 21.3% 940 22.2% Stevanato Group has continued staff development plans in 2021 to support the company’s growth program. In 2021, new hires included 1,173 people, of which almost 41% were women. The Group hiring rate was around 25.2% and the new hires included 508 people in the under 30 age group, 558 in the intermediate age group (30-50), while the remaining 107 were over 50 years old. In general, the search in the production area focuses on candidates with a technical and mechanical degree, while for staff positions a three-year degree or master’s degree in Engineering or Biotechnology is required, followed by Mathematics, Chemistry and Economics. The before-mentioned company growth program is part of a broader integrated human resources development plan supporting Stevanato Group’s Vision and business plan. This HR development plan is divided into a series of project activities that cover all areas of Human Resources, including analysis of process efficiency; talent development, career planning and job rotation based on evaluation of performance and potential; targeted training plans and paths for the development of “soft” and “technical” skills through a Stevanato Group Academy; and finally, a Welfare system for Group companies. Human capital 87
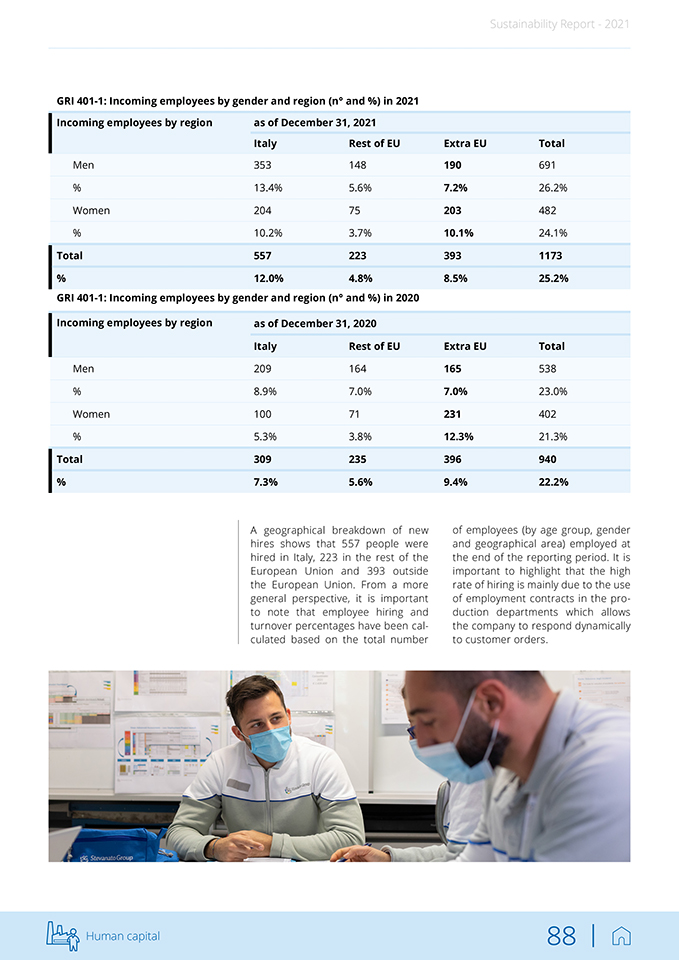
Sustainability Report—2021 GRI 401-1: Incoming employees by gender and region (n° and %) in 2021 Incoming employees by region as of December 31, 2021 Italy Rest of EU Extra EU Total Men 353 148 190 691 % 13.4% 5.6% 7.2% 26.2% Women 204 75 203 482 % 10.2% 3.7% 10.1% 24.1% Total 557 223 393 1173 % 12.0% 4.8% 8.5% 25.2% GRI 401-1: Incoming employees by gender and region (n° and %) in 2020 Incoming employees by region as of December 31, 2020 Italy Rest of EU Extra EU Total Men 209 164 165 538 % 8.9% 7.0% 7.0% 23.0% Women 100 71 231 402 % 5.3% 3.8% 12.3% 21.3% Total 309 235 396 940 % 7.3% 5.6% 9.4% 22.2% A geographical breakdown of new hires shows that 557 people were hired in Italy, 223 in the rest of the European Union and 393 outside the European Union. From a more general perspective, it is important to note that employee hiring and turnover percentages have been cal- culated based on the total number of employees (by age group, gender and geographical area) employed at the end of the reporting period. It is important to highlight that the high rate of hiring is mainly due to the use of employment contracts in the production departments which allows the company to respond dynamically to customer orders. Human capital 88 |
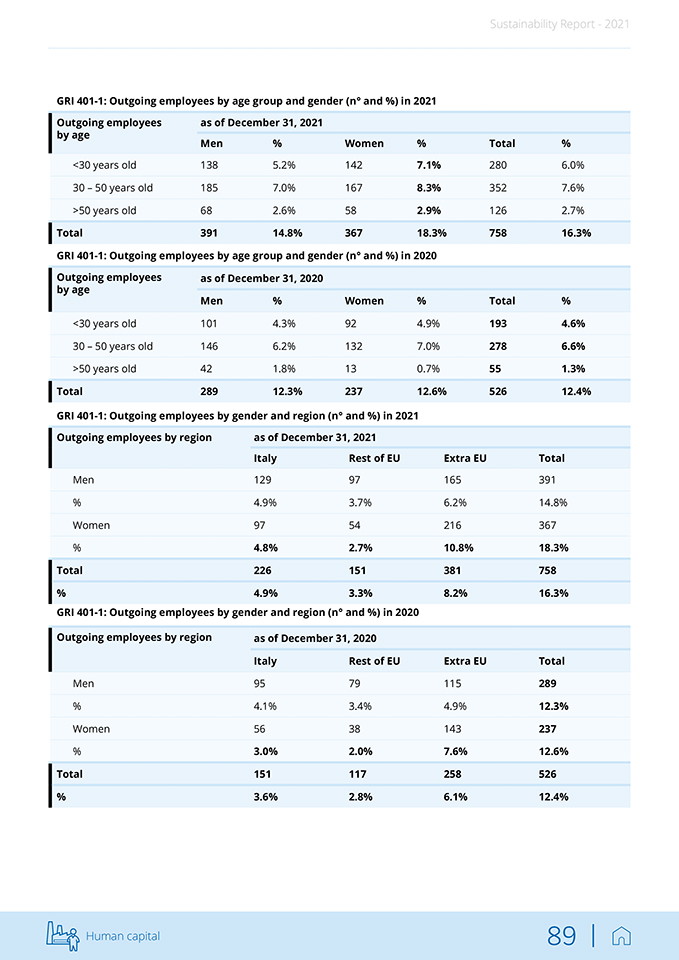
Sustainability Report—2021 GRI 401-1: Outgoing employees by age group and gender (n° and %) in 2021 Outgoing employees as of December 31, 2021 by age Men % Women % Total % <30 years old 138 5.2% 142 7.1% 280 6.0% 30 – 50 years old 185 7.0% 167 8.3% 352 7.6% >50 years old 68 2.6% 58 2.9% 126 2.7% Total 391 14.8% 367 18.3% 758 16.3% GRI 401-1: Outgoing employees by age group and gender (n° and %) in 2020 Outgoing employees as of December 31, 2020 by age Men % Women % Total % <30 years old 101 4.3% 92 4.9% 193 4.6% 30 – 50 years old 146 6.2% 132 7.0% 278 6.6% >50 years old 42 1.8% 13 0.7% 55 1.3% Total 289 12.3% 237 12.6% 526 12.4% GRI 401-1: Outgoing employees by gender and region (n° and %) in 2021 Outgoing employees by region as of December 31, 2021 Italy Rest of EU Extra EU Total Men 129 97 165 391 % 4.9% 3.7% 6.2% 14.8% Women 97 54 216 367 % 4.8% 2.7% 10.8% 18.3% Total 226 151 381 758 % 4.9% 3.3% 8.2% 16.3% GRI 401-1: Outgoing employees by gender and region (n° and %) in 2020 Outgoing employees by region as of December 31, 2020 Italy Rest of EU Extra EU Total Men 95 79 115 289 % 4.1% 3.4% 4.9% 12.3% Women 56 38 143 237 % 3.0% 2.0% 7.6% 12.6% Total 151 117 258 526 % 3.6% 2.8% 6.1% 12.4% Human capital 89 |
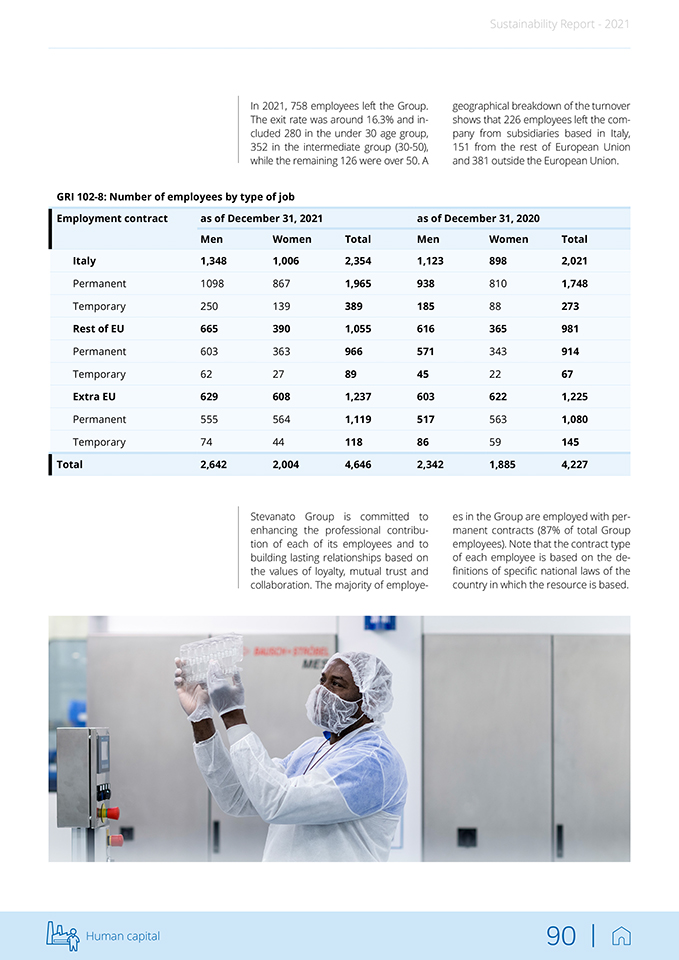
Sustainability Report—2021 In 2021, 758 employees left the Group. The exit rate was around 16.3% and included 280 in the under 30 age group, 352 in the intermediate group (30-50), while the remaining 126 were over 50. A geographical breakdown of the turnover shows that 226 employees left the company from subsidiaries based in Italy, 151 from the rest of European Union and 381 outside the European Union. GRI 102-8: Number of employees by type of job Employment contract as of December 31, 2021 as of December 31, 2020 Men Women Total Men Women Total Italy 1,348 1,006 2,354 1,123 898 2,021 Permanent 1098 867 1,965 938 810 1,748 Temporary 250 139 389 185 88 273 Rest of EU 665 390 1,055 616 365 981 Permanent 603 363 966 571 343 914 Temporary 62 27 89 45 22 67 Extra EU 629 608 1,237 603 622 1,225 Permanent 555 564 1,119 517 563 1,080 Temporary 74 44 118 86 59 145 Total 2,642 2,004 4,646 2,342 1,885 4,227 Stevanato Group is committed to enhancing the professional contribution of each of its employees and to building lasting relationships based on the values of loyalty, mutual trust and collaboration. The majority of employe- es in the Group are employed with permanent contracts (87% of total Group employees). Note that the contract type of each employee is based on the definitions of specific national laws of the country in which the resource is based. Human capital 90 |
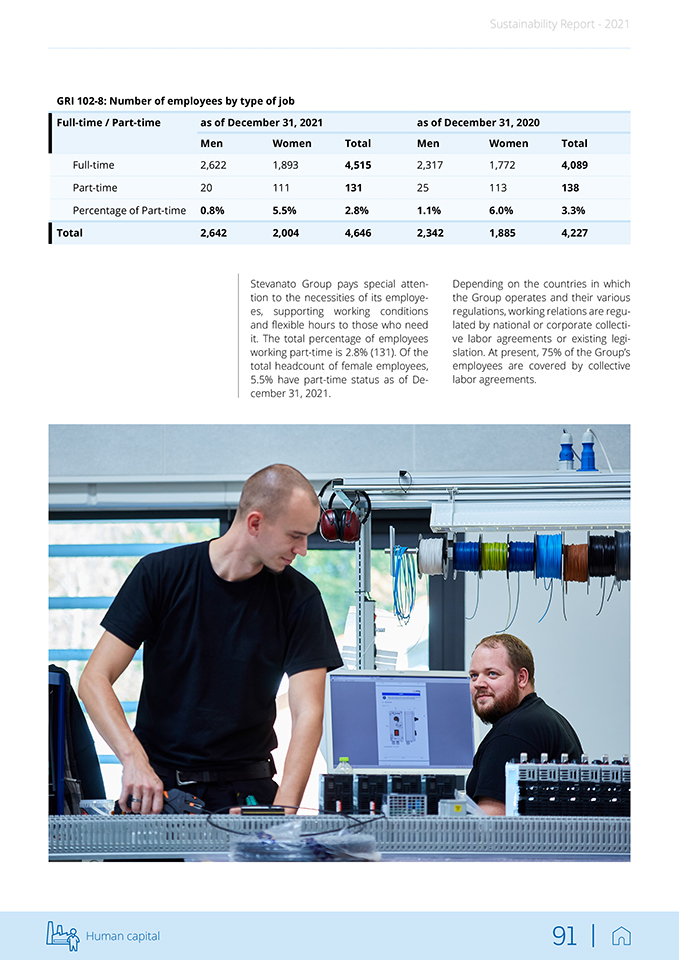
Sustainability Report—2021 GRI 102-8: Number of employees by type of job Full-time / Part-time as of December 31, 2021 as of December 31, 2020 Men Women Total Men Women Total Full-time 2,622 1,893 4,515 2,317 1,772 4,089 Part-time 20 111 131 25 113 138 Percentage of Part-time 0.8% 5.5% 2.8% 1.1% 6.0% 3.3% Total 2,642 2,004 4,646 2,342 1,885 4,227 Stevanato Group pays special attention to the necessities of its employees, supporting working conditions and flexible hours to those who need it. The total percentage of employees working part-time is 2.8% (131). Of the total headcount of female employees, 5.5% have part-time status as of De-cember 31, 2021. Depending on the countries in which the Group operates and their various regulations, working relations are regulated by national or corporate collective labor agreements or existing legislation. At present, 75% of the Group’s employees are covered by collective labor agreements. Human capital 91 |

Sustainability Report—2021 5.2 Employee management and development Stevanato Group is focused on providing a work environment that is free from physical, verbal and sexual harassment, where all employees are respected and have the opportunity to reach their potential and contribute to the success of the company. The Group respects the right to freedom of association and all applicable laws relating to the minimum age of employment. With a worldwide presence, Stevanato Group employs more than 4,600 people who share its commitment towards customer satisfaction and patient well-being. Joining SG means becoming part of a group of highly dedicated people who are committed to developing innovative solutions to complex problems for its customers across the pharmaceutical industry. Departments, positions and opportunities are related to: • Corporate Functions with a focus on Finance, Administration and Control, Human Resources and Supply Chain; • Marketing & Sales as part of the Group’s commercial team; • R&D with a dedicated team of engineers and scientific experts; • Engineering with a focus on experts in Technologies; • Operations & Quality for the development of high-quality products for the pharmaceutical market. Human capital 92 |

Sustainability Report—2021 In order to correctly manage human resources, and in consideration of the location of all group companies, Steva-nato Group has structured a human resources management system that combines a number of systems and processes to ensure the easy management of human resources, business processes and data. With this, the Group can combine several fundamental HR functions, such as storing employee data, managing payroll, recruitment, benefits administration, time and attendance, employee performance management, and competency tracking. Stevanato Group’s remuneration policy is designed to reward fairly, following the guiding principle of meri-tocracy. This is ensured by constant monitoring of the total compensation provided by the markets and by aligning to its professional model which takes all positions into account: tech- nical, professional and managerial. The remuneration policy pays individuals based on qualitative measures, such as pre-defined steps connected to their performance in line with the Group’s values, and on quantitative measures such as the achievement of specific KPIs. In 2020, Stevanato launched a new Performance Development Process (PDP) based on the Group Mission and Vision. There are two categories of PDP in Ste-vanato Group: • Managerial SG PDP: evaluated on a competency model made up of 12 competencies and 5 Guiding Principles • Professional SG PDP: evaluated on a competency model made up of 6 competencies and verification of the employee’s Consistency with SG Guiding Principles Human capital 93 |
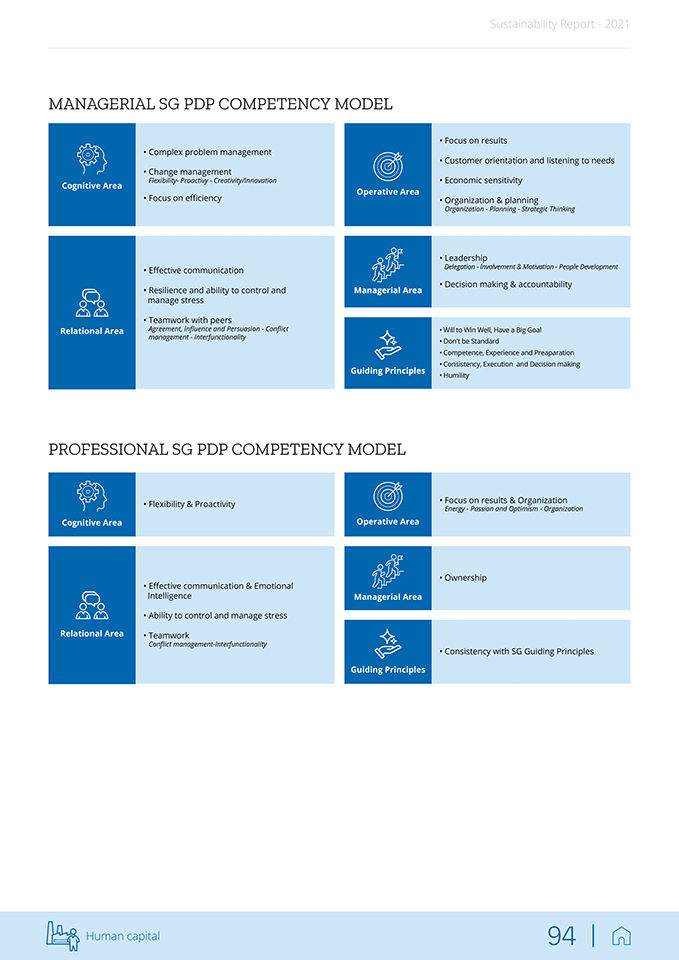
Sustainability Report—2021 MANAGERIAL SG PDP COMPETENCY MODEL • Complex problem management • Change management Flexibility- Proactivy—Creativity/Innovation Cognitive Area • Focus on efficiency • Effective communication • Resilience and ability to controI and manage stress • Teamwork with peers Relational Area Agreement, lnfluence and Persuasion—Conflict management—Interfunctionality Operative Area • Focus on results • Customer orientation and listening to needs • Economic sensitivity • Organization & planning • Leadership Delegation—lnvolvement & Motivation—People Development • Decision making & accountability Managerial Area • Will to Win Well, Have a Big Goal • Don’t be Standard • Competence, Experience and Preaparation • Consistency, Execution and Decision making Guiding Principles • Humility PROFESSIONAL SG PDP COMPETENCY MODEL • Flexibility & Proactivity Cognitive Area • Effective communication & Emotional Intelligence • Ability to control and manage stress Relational Area • Teamwork Conflict management-lnterfunctionality • Focus on results & Organization Energy—Passion and Optimism—Organization Operative Area • Ownership Managerial Area • Consistency with SG Guiding Principles Guiding Principles Human capital 94 |

Sustainability Report—2021 THE PERFORMANCE DEVELOPMENT PROCESS Defining what it can be expected from people and how they can contribute to the achievement of SG’s goals. The Fuel purposes are setting specific, measurable, achievable, reliable and timely objectives (following SMART guidelines) in Performance line with role related needs and business needs; providing individuals with a clear vision of the expectations related to their role in line with company objectives and priorities and finally fostering the creation of a culture oriented toward Setting Goal responsible people development. Seeing, reinforcing and supporting the orts made by individuals in the pursuit of those same objectives throughout the year, modifying priorities and resources according to the upcoming changes. The purposes are providing the employee and manager the opportunity to conduct an open and honest communication based on facts, which will facilitate improvement, development and growth. Discussion Mid-year See Performance It is about seeing, reinforcing and supporting the orts made by individuals in pursuit of those same objectives throughout the year by gathering input from those who work closely with the individual and/or might provide relevant feedback. The purposes are broadening the scope of Performance Evaluation by involving directly additional managers and/or peers into the Evaluation process to gather significant information that improves the overall Feedback Evaluation; increasing objectivity of the Performance Evaluation and finally involving key stakeholders that might provide relevant Feedback Collection feedback on the individual’s performance. Recognizing, assessing and evaluating the individual performance observed throughout the year. The purposes are recognizing the performance of the year; evaluating the Employee based on actual behaviors and results instead of relying on assumptions, thus reducing the subjectivity and rating the objectives set at the beginning of the year and Performance the relative Competency Model. Recognize Evaluation Performance Discussing the Performance Evaluation with the individual through an open and transparent dialogue. The purposes are providing an opportunity to foster motivation and engagement by providing feedback on yearly performance and setting expectations for next months and finally providing a formal occasion to reinforce positive behaviors and correct Feedback Session ineective ones. Another key aspect contributing to the professional development of employees is training provided by the Group. Stevanato Group considers ongoing employee training an essential part of the company philosophy. The development of specialized skills and abilities not only maintains the Group’s competitive advantage, but also ensures that its customers receive technical solutions in line with their needs both in terms of quality and innovation. To this end, a few years ago Stevanato Group created a specific function called “SG Academy” which manages and coordinates training activities at the Corporate Level. In 2020, SG Academy became “SG Academy & Change Management”, a reference function for the launch of projects aimed at supporting critical phases of change. SG Academy designs and launches training initiatives ranging from technical workshops to “Soft Skills” development sessions. The Academy promotes a “One Group” approach encouraging knowledge acquisition and the adoption of the Group’s Values and Guiding Principles. Training is a fundamental process which starts at the very beginning of an employee’s professional career. In addition, EHS training is provided during the probation period and repeated with the frequency and manner prescribed by law. Based at the Group headquarters, SG Academy is responsible for monitoring global training activities and managing and designing corporate training. Furthermore, the department conducts an annual assessment of training needs and requirements in order to define a training plan for the year which strongly correlates with Budget guidelines and constraints. At the local level, the process is directly coordinated by the Local HR Manager in alignment with SG Academy. The training needs assessment process is directly managed Human capital 95 |

Sustainability Report—2021 by SG Academy and relies on different sources, such as: individual meetings with Managers to discuss the specific needs of collaborators; analysis of Performance Evaluation results to identify the priorities to be addressed; alignment with HR Development and HR Business Partners to follow up on the most critical areas of improvement and to support the implementation of specific development plans. Locally, the HR Manager identifies specific needs based on feedback from local Managers and shares them with the SG Academy. The implementation of the Local Training Plan is directly managed by the Local HR Manager in constant coordination with the SG Academy team. As mentioned before, the assessment of training needs provides a framework for the annual Training Plan, which includes both Corporate and Local initiatives. In particular, the Academy foresees three main areas: • Soft Skills Academy: this area is dedicated to the development of soft skills for Stevanato Group employees. The training is based on a structured Catalog designed and launched by SG Academy, and is dedicated to the development of competencies considered fundamental by the Company and directly linked to the Performance Development Process. Professional Academies: the main objectives of this area include fostering knowledge-sharing within the company and developing specific cross-competencies. For example, a “Finance for non-Finance” training module, which teaches financial skills to people who work in other areas. The courses are structured with particular attention to the level of prior knowledge, which is measured through a specific initial test, and to the type of target population, creating both basic modules and more specific advanced modules. Local Education: this area is dedicated to the locally-managed training programs such as language training and technical-specific initiatives dedicated to a restricted population or individual resource. Human capital 96 |

Sustainability Report—2021 Across the Group companies, training is delivered in different ways including classroom lessons, on-the-job training, video lessons and distance education like virtual classrooms. On-the-job training is done in the workplace/workstation while the employee is performing the actual job. During 2021, the Group provided 14,393 hours of employee training related to EHS and mandatory by law, and 46,472 hours of training on the Quality Management System in compliance with ISO 15378, which is explained in detail in the sub-chapter below. The Group’s remaining training activities classified as “not mandatory” amount to a total of 46,924 hours and include training in induction, soft skills, linguistic skills, technical skills, change management initiatives and job instructions. In total, SG provided 107,789 hours of training during 2021. The Group is working to further structure data collection for trainings and attendance reports in order to meet GRI Standards in the coming years. Quality Training Company training also underlines the importance of Quality for all Stevanato Group departments. Each employee is made aware of the Quality Policy and relevant Quality Objectives related to the Quality Management System, including the benefits of improved performance as well as the implications of non-conformity with the requirements. In compliance with ISO Standards and the principles of Good Manufacturing Practices, Quality training is regularly provided and mapped for all employees. In relation to Good Manufacturing Practices, the training includes the risk of contamination and cross-contamination, potential hazards to the end-user and/or patient and the impact of any deviations from specified procedures, processes or specifications on product quality or to the end-user. Particular attention is dedicated to the training of personnel involved with the manufacturing of sterile components. In addition, specific training in micro-biological and particulate contamination and the potential risk of contamination to the patient is provided to select employees. Change Management Change Management is the application of a structured process and set of tools for leading the people-side of change in order to achieve a desired outcome. “SG Academy & Change Management” has become the point of reference for the launch of programs promoting important phases of transformation. In particular, these initiatives aim to support change in terms of Mindset and leverage the involvement of people. The Change Management programs are different and range from supporting the transition of important organizational changes to guiding important evolutions, such as digitization. Initiatives conducted in in 2021 included the Group Change Management Program, the CTO Integration Journey and the One Engineering Program. All of these initiatives were aimed at fostering Customer Centricity and integration, and supporting SG in becoming a “Best in Class” provider of integrated solutions. Other Corporate Initiatives As part of SG’s “One Group” approach, the Academy and HR Team focus on initiatives that contribute to the sharing of knowledge and the adoption of the Company Values and Guiding Principles. Initiatives range from the definition and launch of dedicated Programs (such as a Mentorship Program), to the creation and release of specific tools that integrate the Group’s Values and Guiding Principles into daily work life. Stevanato Group believes that its Values and Guiding Principles are strongly connected with Business Objectives and, as such, are key to reaching SG’s ambitious Goals. Human capital 97 |

Sustainability Report—2021 Stevanato Group Welfare In regards to company welfare and employee well-being, Stevanato Group is currently implementing a global corporate welfare system in all its plants worldwide. From the end of 2020 and onwards, Stevanato Group has expanded the welfare plan at its facilities in Piombino Dese, adding further support measures and devoting special attention to employees in difficult situations, including issues caused by the pandemic. The agreement was designed in collaboration with the facility’s Trade Union and covers several interesting points. First, in the event of periods of absence that exceed those provided by law due to a serious and ongoing illness requiring life-saving treatment, the employee will retain his/her job until recovery. Additionally, permits have also been granted to take care of disabled minor children, and days of absence and flexible hours have been increased for the care of ill children up to 12 years of age. The agreement also provides assistance to families and two new benefits have been activated: • Fragility Bonus: in order to support employees with disabled children, an individual budget of 1,000 Euro has been assigned to select employees. This bonus increases to 3,000 Euro for employees that have disabled children under 4 years of age. • Birth Bonus: an individual budget of 500 Euro has been assigned to those employees that gave birth to a child during 2021. Finally, in support of new mothers, maternity leave has been extended up to 18 months after the birth of their child. Stevanato Group provides support and helps new mothers return to the wor-kplace with ad hoc designed paths. Employees of each facility are eligible for health benefits provided by the company or the government. In particular, in the Balda, USA facilities and Ompi of Brazil, all employees are eligible for health care benefits for which Stevanato Group pays a certain percentage and the employees pay their own share depending on the type of plan they choose. In addition, Balda Precision has provided an additional 24 hours of sick time to support employees during the Covid pandemic. Medical Glass provides additional pension insurance to all employees, whereby the amount of the allowance depends on the length of employment according to agreements with the Trade Union. Regarding the services offered to employees, no distinction is made in relation to contract type (part-time/ full-time) but benefits vary according to geographic area and applicable regulations. In Ompi North America all employees have: • Dining service during their shift (subsidized); • Transportation for operative staff; • Food vouchers; • Life insurance to protect workers’ families; • School support to promote education for children of employees. Partnerships with training institutes and universities Stevanato Group is strongly committed to discovering and cultivating employees of the future. To this end, it forges close collaborations with schools and universities to establish school-work alternation and internship opportunities. The Group regards these esteemed partnerships as a pivotal element of its sustainability strategy, as it directly improves students’ career prospects and their placement in the labor market. Human capital 98 |

Sustainability Report—2021 Over the years, Stevanato Group has initiated various collaborations with schools and universities in the areas where the company’s plants are located. Locally-based partnerships are the best way to promote the company and attract talent. In this context, Stevanato Group has specifically launched a Graduate Program for new graduates. The 18-month program includes two job rotations within two main functions that are based on the graduate’s educational background: Sales & Marketing, R&D and Operations. A tutor is assigned to the resource and supports their development within Stevanato Group through job training and classes aimed at developing soft skills. At the end of the period, the resource is assigned a role that best matches the evaluation results .. Human capital 99 |

Human capital 99 | 5.3 Occupational Health & Safety For Stevanato Group, health and safety work conditions include prevention of physical and mental harm and promotion of employee health. All business activities are carried out in line with the occupational health and safety regulations in force, as well as the requirements of the Code of Ethics and the Group’s EHS Policy and Guidelines. Analysis and Risk Assessment are regularly performed to detect the presence of any hazards in the workplace, to identify and assess risks, and to engage employees in the development, implementation and performance evaluation of occupational health and safety management systems. In accordance with the EHS policy, Stevanato Group aims to ensure the health and safety of its employees, customers, users, suppliers, partners and visitors. The EHS Policy statement has been issued by the Corporate CEO and will be reissued as necessary and communicated to all individuals and stakeholders during audits or working visits. It has also been published in targeted communication areas and on the corporate website. The core of Stevanato’s EHS Policy regards Top Management’s EHS philosophy, commitment and expectations, thus providing a visible guide for the entire organization. In line with this policy and in compliance with applicable legal requirements in terms of health and safety, Stevanato encourages its entire organization and stakeholders to act according to the highest standards. Doing so helps anticipate risks and identify preventive actions that contribute to the reduction of possible injuries or occupational illnesses. Human capital 100 |
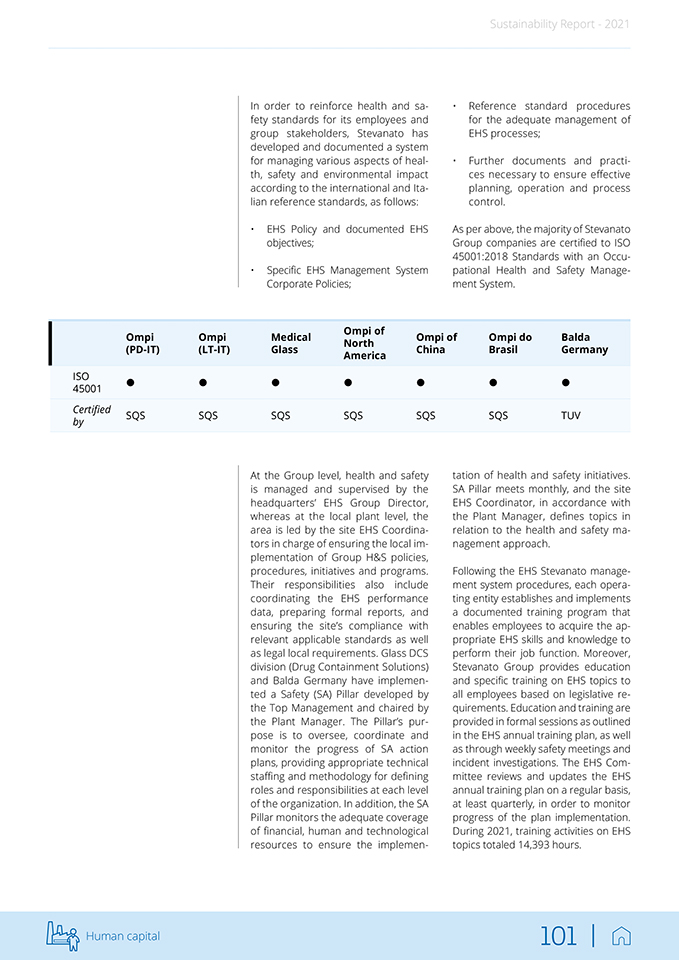
Sustainability Report—2021 In order to reinforce health and safety standards for its employees and group stakeholders, Stevanato has developed and documented a system for managing various aspects of health, safety and environmental impact according to the international and Ita-lian reference standards, as follows: • EHS Policy and documented EHS objectives; • Specific EHS Management System Corporate Policies; • Reference standard procedures for the adequate management of EHS processes; • Further documents and practices necessary to ensure effective planning, operation and process control. As per above, the majority of Stevanato Group companies are certified to ISO 45001:2018 Standards with an Occupational Health and Safety Management System. Ompi of Ompi Ompi Medical Ompi of Ompi do Balda North (PD-IT) (LT-IT) Glass China Brasil Germany America ISO 45001ï,~ï,~ï,~ï,~ï,~ï,~ï,~ Certified SQS SQS SQS SQS SQS SQS TUV by At the Group level, health and safety is managed and supervised by the headquarters’ EHS Group Director, whereas at the local plant level, the area is led by the site EHS Coordinators in charge of ensuring the local implementation of Group H&S policies, procedures, initiatives and programs. Their responsibilities also include coordinating the EHS performance data, preparing formal reports, and ensuring the site’s compliance with relevant applicable standards as well as legal local requirements. Glass DCS division (Drug Containment Solutions) and Balda Germany have implemented a Safety (SA) Pillar developed by the Top Management and chaired by the Plant Manager. The Pillar’s purpose is to oversee, coordinate and monitor the progress of SA action plans, providing appropriate technical staffing and methodology for defining roles and responsibilities at each level of the organization. In addition, the SA Pillar monitors the adequate coverage of financial, human and technological resources to ensure the implemen- tation of health and safety initiatives. SA Pillar meets monthly, and the site EHS Coordinator, in accordance with the Plant Manager, defines topics in relation to the health and safety management approach. Following the EHS Stevanato management system procedures, each operating entity establishes and implements a documented training program that enables employees to acquire the appropriate EHS skills and knowledge to perform their job function. Moreover, Stevanato Group provides education and specific training on EHS topics to all employees based on legislative requirements. Education and training are provided in formal sessions as outlined in the EHS annual training plan, as well as through weekly safety meetings and incident investigations. The EHS Committee reviews and updates the EHS annual training plan on a regular basis, at least quarterly, in order to monitor progress of the plan implementation. During 2021, training activities on EHS topics totaled 14,393 hours. Human capital 101 |

Sustainability Report—2021 Participation and consultation in the development, implementation and evaluation of the occupational health and safety management system for all employees and their representatives is through: • their involvement and prior consultation regarding the identification of hazards, risk assessment, control measures, preventive measures and accident analysis; • their involvement in the development and revision of EHS policies, objectives and goals; • periodical meetings held in accordance with the frequency and method of the local legislation. Every day, before starting work or a new job, employees inspect their work stations to identify potential risks. Each hazardous condition is reported to the organization via specific reporting formats called TAG. Follow-up activities and corrective-action tracing are addressed by the Improvement Team on Safety which is led by department Supervisors. Supervisors and Shift Leaders must issue a monthly safety inspection of their responsibility areas by reporting non-conformities on specific checklists. The checklists cite risks, potential hazards, observable unsafe acts and compliance issues, which are reported and examined periodically by Local Top Management. Incidents are reported, reviewed and investigated to a level commensurate with the actual or potential consequence of the occurrence. In case of incidents with no serious consequences, a formal investigation is led by the department leader and supported by the site EHS Coordinator. When the severity of the consequences requires leadership to verify the accuracy of the investigation, Top Management is involved. Corrective actions are taken in order to avoid the repetition of the same accident. The site SA Pillar Team reviews inspections and corrective actions monthly to verify their consistency and ensure the completion of the corrective actions. All injuries with prognosis days are reviewed by HQ EHS dept. for leadership’s involvement and confirm the consistency of corrective actions, in addition to sharing lessons learned and best practices at the corporate level. Following significant health injuries, the HR department, along with the local EHS Coordinator, supports the injured person’s return to work through a re-entry program. Human capital 102 |

Sustainability Report—2021 The program may include medical examination by the company doctor, temporary assignment to a less difficult task and a recovery plan for the previous job role. In relation to fatality and serious injury management, the Stevanato Group Fatality & Serious Injury Review Board, chaired by a representative of the Chairman and CEO, and composed by several executive employees both at the Group and local level, reviews: • all employee workplace fatalities and serious injuries occurring within the operative unit; • work-related fatalities suffered by contractor personnel working directly for Stevanato Group at a Group facility or job site; • other safety-related incidents (e.g., “serious near misses”). To facilitate the reporting of occurrences by employees to line management, the SA Pillar team has implemented a TAG communication system which is clearly posted in working areas or specific departments. This system collects reports concerning unsafe acts, unsafe behaviors and near misses. At least once a week, the department Improvement Team led by supervisors, and composed of a representative of other supporting functions, analyzes events reported in order to identify root causes and assess opportunities for improvement or corrective actions. The occurrences are reported via an Improvement Plan Actions List, which is reviewed and analyzed to ensure that corrective and preventive actions are applied. Based on the analysis of risks pertaining to various tasks, the company medical service visits the workplace periodically and prescribes a medical protocol for the residual risks, which is cyclically reviewed. In some Group entities6, employees have access to additional healthcare insurance that includes agreements with third-party private medical clinics. In fact, as part of SG’s corporate welfare program, employees have supplementary health coverage (reimbursement of expenses + various free services). Furthermore, in compliance with legislative requirements, all Stevanato Group companies provide periodic mandatory medical visits carried out by an assigned doctor who assesses employee health and job suitability. Stevanato Group companies organize initiatives aimed at promoting health through diverse medical care services that are free-of-charge. Some health promotion campaigns have been carried out locally in order to promote awareness and good practice, including flu vaccination campaigns, cardiovascular disease prevention campaigns and breast cancer prevention campaigns. The table below shows the main health promotion initiatives carried out in 2021 around the world. 6. The companies are: Nuova Ompi, Spami, Balda Medical, Balda USA, Innoscan, SVM, Ompi do Brasil, Ompi Pharmaceutical Packing Technology and Stevanato Group NAS. Human capital 103 |
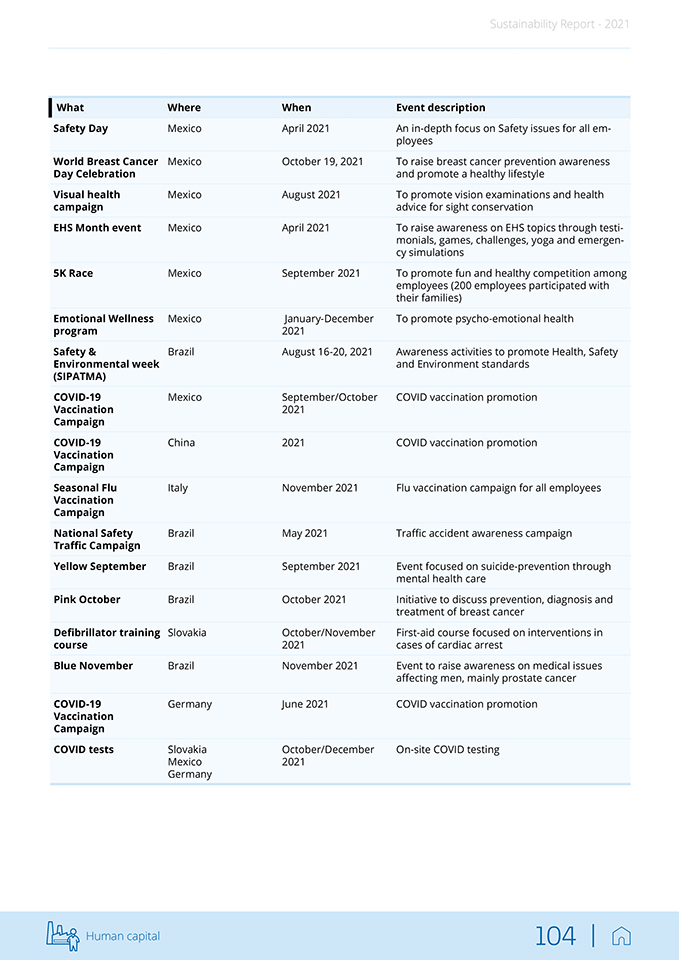
Sustainability Report—2021 What Where When Event description Safety Day Mexico April 2021 An in-depth focus on Safety issues for all employees World Breast Cancer Mexico October 19, 2021 To raise breast cancer prevention awareness Day Celebration and promote a healthy lifestyle Visual health Mexico August 2021 To promote vision examinations and health campaign advice for sight conservation EHS Month event Mexico April 2021 To raise awareness on EHS topics through testimonials, games, challenges, yoga and emergency simulations 5K Race Mexico September 2021 To promote fun and healthy competition among employees (200 employees participated with their families) Emotional Wellness Mexico January-December To promote psycho-emotional health program 2021 Safety & Brazil August 16-20, 2021 Awareness activities to promote Health, Safety Environmental week and Environment standards (SIPATMA) COVID-19 Mexico September/October COVID vaccination promotion Vaccination 2021 Campaign COVID-19 China 2021 COVID vaccination promotion Vaccination Campaign Seasonal Flu Italy November 2021 Flu vaccination campaign for all employees Vaccination Campaign National Safety Brazil May 2021 Traffic accident awareness campaign Traffic Campaign Yellow September Brazil September 2021 Event focused on suicide-prevention through mental health care Pink October Brazil October 2021 Initiative to discuss prevention, diagnosis and treatment of breast cancer Defibrillator training Slovakia October/November First-aid course focused on interventions in course 2021 cases of cardiac arrest Blue November Brazil November 2021 Event to raise awareness on medical issues affecting men, mainly prostate cancer COVID-19 Germany June 2021 COVID vaccination promotion Vaccination Campaign COVID tests Slovakia October/December On-site COVID testing Mexico 2021 Germany Human capital 104 |
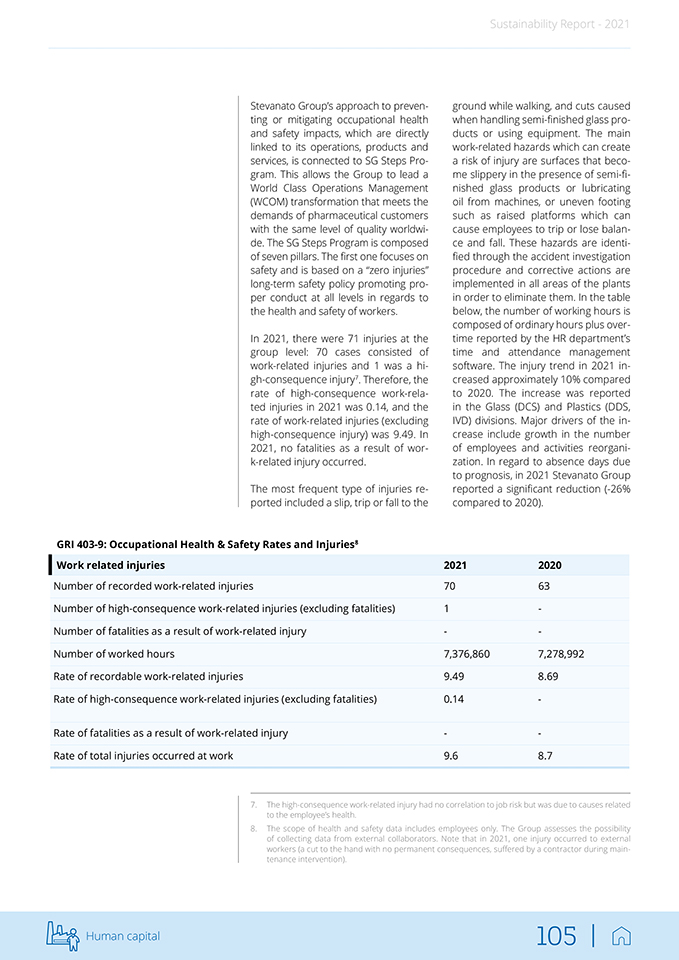
Sustainability Report—2021 Stevanato Group’s approach to preventing or mitigating occupational health and safety impacts, which are directly linked to its operations, products and services, is connected to SG Steps Program. This allows the Group to lead a World Class Operations Management (WCOM) transformation that meets the demands of pharmaceutical customers with the same level of quality worldwide. The SG Steps Program is composed of seven pillars. The first one focuses on safety and is based on a “zero injuries” long-term safety policy promoting proper conduct at all levels in regards to the health and safety of workers. In 2021, there were 71 injuries at the group level: 70 cases consisted of work-related injuries and 1 was a high-consequence injury7. Therefore, the rate of high-consequence work-related injuries in 2021 was 0.14, and the rate of work-related injuries (excluding high-consequence injury) was 9.49. In 2021, no fatalities as a result of work-related injury occurred. The most frequent type of injuries reported included a slip, trip or fall to the ground while walking, and cuts caused when handling semi-finished glass products or using equipment. The main work-related hazards which can create a risk of injury are surfaces that become slippery in the presence of semi-finished glass products or lubricating oil from machines, or uneven footing such as raised platforms which can cause employees to trip or lose balance and fall. These hazards are identified through the accident investigation procedure and corrective actions are implemented in all areas of the plants in order to eliminate them. In the table below, the number of working hours is composed of ordinary hours plus overtime reported by the HR department’s time and attendance management software. The injury trend in 2021 increased approximately 10% compared to 2020. The increase was reported in the Glass (DCS) and Plastics (DDS, IVD) divisions. Major drivers of the increase include growth in the number of employees and activities reorganization. In regard to absence days due to prognosis, in 2021 Stevanato Group reported a significant reduction (-26% compared to 2020). GRI 403-9: Occupational Health & Safety Rates and Injuries8 Work related injuries 2021 2020 Number of recorded work-related injuries 70 63 Number of high-consequence work-related injuries (excluding fatalities) 1 -Number of fatalities as a result of work-related injury —Number of worked hours 7,376,860 7,278,992 Rate of recordable work-related injuries 9.49 8.69 Rate of high-consequence work-related injuries (excluding fatalities) 0.14—Rate of fatalities as a result of work-related injury —Rate of total injuries occurred at work 9.6 8.7 7. The high-consequence work-related injury had no correlation to job risk but was due to causes related to the employee’s health. 8. The scope of health and safety data includes employees only. The Group assesses the possibility of collecting data from external collaborators. Note that in 2021, one injury occurred to external workers (a cut to the hand with no permanent consequences, suffered by a contractor during maintenance intervention). Human capital 105 |
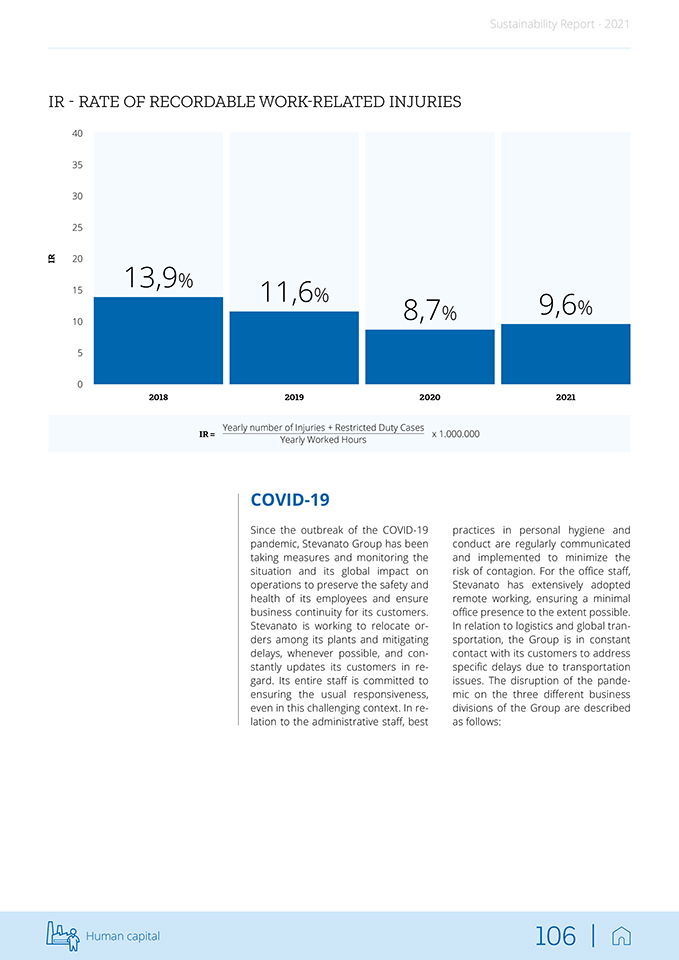
Sustainability Report—2021 IR—RATE OF RECORDABLE WORK-RELATED INJURIES 40 35 30 25 IR 20 13,9% 15 11,6% 8,7% 9,6% 10 5 0 2018 2019 2020 2021 Yearly number of Injuries + Restricted Duty Cases IR = x 1.000.000 Yearly Worked Hours COVID-19 Since the outbreak of the COVID-19 pandemic, Stevanato Group has been taking measures and monitoring the situation and its global impact on operations to preserve the safety and health of its employees and ensure business continuity for its customers. Stevanato is working to relocate orders among its plants and mitigating delays, whenever possible, and constantly updates its customers in regard. Its entire staff is committed to ensuring the usual responsiveness, even in this challenging context. In relation to the administrative staff, best practices in personal hygiene and conduct are regularly communicated and implemented to minimize the risk of contagion. For the office staff, Stevanato has extensively adopted remote working, ensuring a minimal office presence to the extent possible. In relation to logistics and global transportation, the Group is in constant contact with its customers to address specific delays due to transportation issues. The disruption of the pandemic on the three different business divisions of the Group are described as follows: Human capital 106

Sustainability Report—2021 Business segments Description Glass manufacturing In Italy, a critical part of the pharmaceutical and healthcare value chain, Stevanato Group activities activities have always been considered “essential business”. Therefore, the activities never stopped and are still operating without interruption in both its manufacturing plants located in Piombino Dese – Padua and Latina. At its plants in Slovakia, Brazil, Ompi N.A. and China, Stevanato is carrying out production seamlessly. The Group has replicated the preventive measures proven effective since the beginning of the pandemic and will keep them in place according to the local government guidelines, making adjustments based on the level of threat. In Mexico, the Government has decided to maintain social distancing, protective measures and suspension of all non-essential activities including transportation not related to essential needs. Therefore, operations are running on schedule, with no delays. Plastic injection Operations in Germany and the US are running on a regular schedule with no delays. molding activities Stevanato has ramped up the production of diagnostic test plates used globally for early COVID-19 detection. The safety precautions in injection molding plants in the US and Germany remain unaltered and include additional sanitation activities following the World Health Organization guidelines. Engineering activities In plants located in Italy and Denmark dedicated to the production of advanced glass processing, inspection, and assembly equipment for the pharmaceutical industry, the Group has continued its production activities. Stevanato has also intensified the usage of advanced remote assistance tools to support customers for FAT, SAT, and post-sales activities. There are no significant production or delivery delays. Human capital 107 |

Supply chain responsibility 6 Human capital 108

Sustainability Report—2021 6.1 Responsible supply chain & procurement In the definition of its supply chain and procurement practices, Stevanato Group’s supplier selection process and purchasing conditions are inspired by the values and criteria of competitiveness, objectivity, respectability, correctness, impartiality, fair pricing and quality. Procurement processes aim to achieve the maximum competitive advantage for the Group while ensuring fairness and impartiality towards every supplier in possession of the necessary prerequisites. The contractual relations with Stevana- to Group are subject to an initial asses- sment at the time of purchase and are regularly monitored. SG has specified analytical purchasing rules that establi- sh the contractual conditions, gover- ning the purchase of raw materials, se- mi-finished products or preparations and services. The Group’s supply chain is composed of more than five thousand suppliers. Focusing our analysis on suppliers with annual spending higher than 150.000 € (or equivalent in local currency), it is possible to note that more than 86% of the total expense applies to only 379 suppliers. These select suppliers represent just 8% of the total number (5,001). In particular, the top 10 Steva- nato Group suppliers account for 25% of the total procurement amount. Pharmaceutical quality & regulatory standards set specific requirements for products and services in the indu- stry. Stevanato Group works incessant- ly to meet such requirements. Supply chain 109 |

Supply chain 109 | Stevanato Group carries out specific audits in order to test the quality of supply products. The audits are defined by specific procedures designed by Stevanato Procurement functions. These procedures classify all suppliers under an “A-B-C-D” rating, according to the characteristics of the supplied materials or/and services. The A category includes the most important and fundamental resources for Group business operations. Therefore, they are subject to the strictest audit procedures including the analysis of a series of suitability requirements in relation to technological and production capacity, overall quality of processes and products, possession of quality certificates, and corporate and financial position. Under the D category, less important resources are included and more moderate audit procedures are carried out via a supplier questionnaire. Overall, the focus of Stevanato Group Procurement employees is to verify all quality aspects of its suppliers, including documentation management, production processes and relative control. Stevanato Group is also audited by its own corporate customers. CLOUD COMPUTING AND REMOTE ASSISTANCE Stevanato Group has strengthened its collaboration with Microsoft Italy through the use of new technologies designed to aid the pharmaceutical industry in overcoming challenges caused by the pandemic. Thanks to mixed reality, it is now possible to complete virtual audits of quality, health and safety systems, and conduct remote factory acceptance tests (FATs) on equipment. A Virtual Audit plan was launched using the Microsoft HoloLens2 holographic headset with integrated Dynamics Remote Assist. This allows technicians to show customers the installation and processes, including production and lab testing, remotely. It is also possible to perform a remote quality system audit to ascertain that the installation complies with required standards and customer needs. In addition, the HoloLens headset is capable of performing the Virtual FAT (Factory Acceptance Test) — the final pre-delivery inspection that assures the remotely connected customer that the equipment is functioning properly. While wearing the headset, a technician can demonstrate machine components, start it up and share documentation. Remote acceptance of equipment for the inspection of drugs and vaccines makes it possible to accelerate delivery to pharmaceutical companies, a factor that is critical in this period when the company has to manage large volumes on a short time frame. In 2021, Stevanato Group began requiring both new and existing suppliers to adhere to its Code of Conduct. Adherence ensures compliance with existing laws, loyalty, professional rigor and correctness as well as respect for the environment, human rights and workers’ rights. Any conduct that differs from these principles could result in the termination of the business relationship, or be a precluding factor for additional collaboration. Stevanato Group requires its suppliers and service providers (in terms of legal com- pliance) to comply with the statutory requirements of the applicable legal system and with the recognized international standards of ethical conduct. In regards to respect for the basic human rights of employees, the Code is focused on the promotion of equal opportunities in terms of skin color, race, nationality, social background, possible disability, sexual orientation, political or religious conviction, sex or age, and finally, with respect to personal dignity, privacy and the rights of each individual. Supply chain 110 |

With regards to the prohibition of child labor, the Group is committed to ensuring that its suppliers recognize and respect the rights of children and comply with the Conventions 138 and 182 of the International Labor Organization (ILO) on the restriction of child labor. In terms of the safety of suppliers’ employees, the Code emphasizes the importance of taking responsibility for all staff, ensuring the best possible pre-cautionary measures against accidents and providing regular training on health and safety issues. Finally, the last part of the Code of Conduct is related to environmental protection. In this section, the Group requires all suppliers: to act in accordance with the applicable statutory and international standards regarding environmental protection, to avoid environmental pollution through constant improvements in environmental protection protocols, and to set up an environmental management system according to ISO 14001. Presently, SG is not forcing or measuring the sustainability developments of its suppliers connected to ESG aspects. However, it recognizes the importance of this matter and intends to improve its valuation, giving more weight to sustainability values. In fact, the Group’s priority objective is to make the supplier selection system more robust in order to carry out targeted audits that also take environmental and social aspects into account. Sustainability Report—2021 In this context, in 2021 Stevanato Group started a collaboration with EcoVadis, a provider of business sustainability ratings, intelligence and collaborative performance improvement tools for the global supply chain. With EcoVadis’ support, Steva-nato Group is mapping its supplier base by ESG topics with the goal of monitoring their environmental and social responsibility and pushing for corrective actions where needed. This sustainable sourcing project includes the integration of social, ethical and environmental performance factors in the supplier selection process. EcoVa-dis will analyze the first few hundred Stevanato Group suppliers and provide an independent rating on ESG aspects. The project aims to reduce possible ESG sourcing risks in developed and developing Countries as the Group considers the expansion of its supply chain. It will also help SG meet the growing expectations of its stakeholders by ensuring and overseeing its suppliers’ environmental, social and ethical practices. Stevanato Group’s goal was to reach a target of 100 rated suppliers, which it greatly exceeded with a total of 114 suppliers assessed. In 2021, EcoVadis rated approximately 42% of SG’s suppliers and an additional 9% of suppliers have accepted joining the project and are under review. Supply chain 111 |
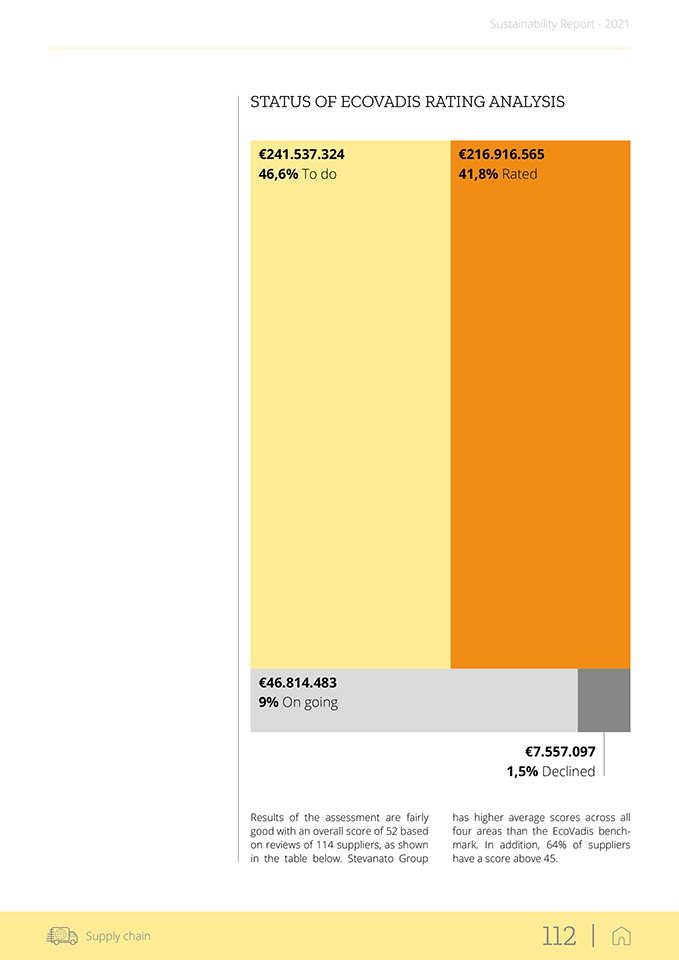
Sustainability Report—2021 STATUS OF ECOVADIS RATING ANALYSIS €241.537.324 46,6% To do €46.814.483 9% On going Results of the assessment are fairly good with an overall score of 52 based on reviews of 114 suppliers, as shown in the table below. Stevanato Group €216.916.565 41,8% Rated €7.557.097 1,5% Declined has higher average scores across all four areas than the EcoVadis benchmark. In addition, 64% of suppliers have a score above 45. Supply chain 112 |
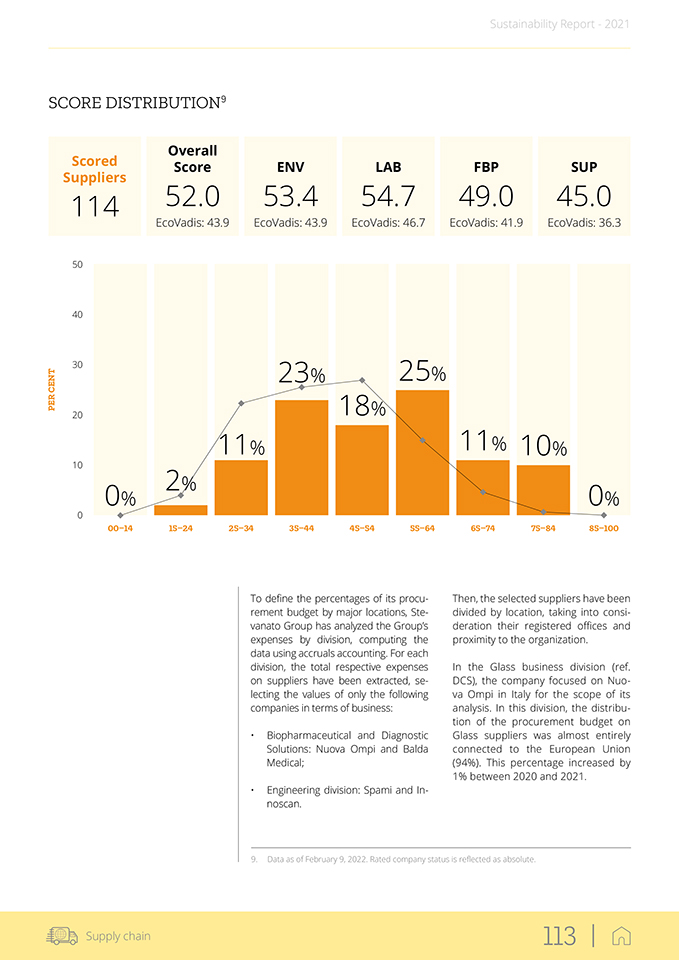
Sustainability Report—2021 SCORE DISTRIBUTION9 Scored Suppliers 114 50 40 30 CENT PER 20 10 0% 0 00–14 Overall Score ENV LAB 52.0 53.4 54.7 EcoVadis: 43.9 EcoVadis: 43.9 EcoVadis: 46.7 23% 25% 18% 11% 2% 15–24 25–34 35–44 45–54 55–64 To define the percentages of its procurement budget by major locations, Ste-vanato Group has analyzed the Group’s expenses by division, computing the data using accruals accounting. For each division, the total respective expenses on suppliers have been extracted, selecting the values of only the following companies in terms of business: • Biopharmaceutical and Diagnostic Solutions: Nuova Ompi and Balda Medical; • Engineering division: Spami and In-noscan. FBP SUP 49.0 45.0 EcoVadis: 41.9 EcoVadis: 36.3 11% 10% 0% 65–74 75–84 85–100 Then, the selected suppliers have been divided by location, taking into consideration their registered offices and proximity to the organization. In the Glass business division (ref. DCS), the company focused on Nuo-va Ompi in Italy for the scope of its analysis. In this division, the distribution of the procurement budget on Glass suppliers was almost entirely connected to the European Union (94%). This percentage increased by 1% between 2020 and 2021. 9. Data as of February 9, 2022. Rated company status is reflected as absolute. Supply chain 113 |
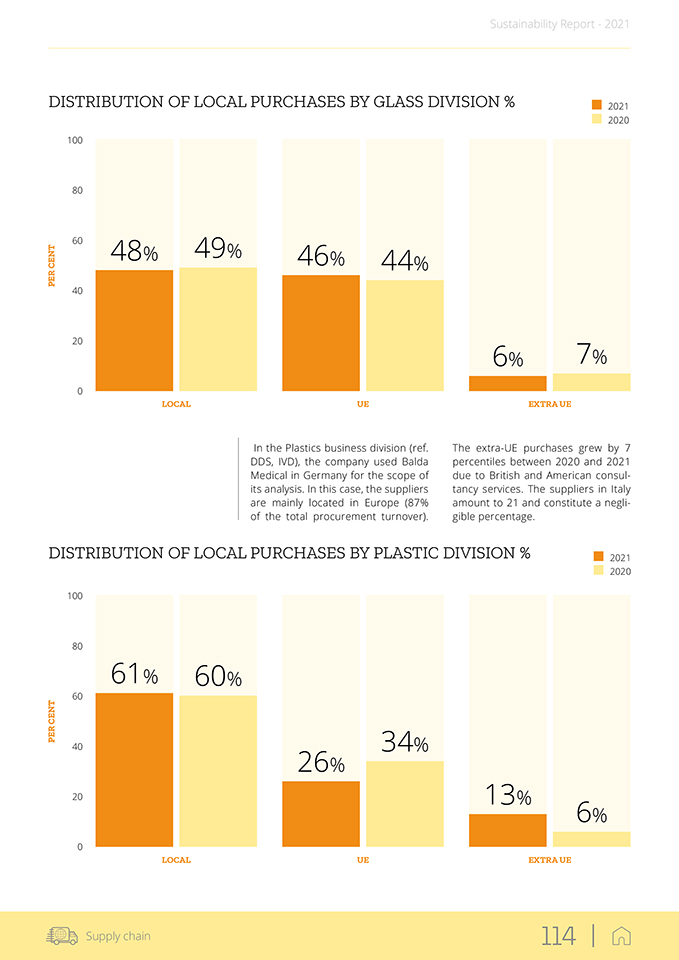
Sustainability Report—2021 DISTRIBUTION OF LOCAL PURCHASES BY GLASS DIVISION % 2021 2020 100 80 60 48 49% CENT % 46% PER 44% 40 20 7 6% % 0 LOCAL UE EXTRA UE In the Plastics business division (ref. DDS, IVD), the company used Balda Medical in Germany for the scope of its analysis. In this case, the suppliers are mainly located in Europe (87% of the total procurement turnover). The extra-UE purchases grew by 7 percentiles between 2020 and 2021 due to British and American consultancy services. The suppliers in Italy amount to 21 and constitute a negligible percentage. DISTRIBUTION OF LOCAL PURCHASES BY PLASTIC DIVISION % 2021 2020 100 80 61% 60% 60 CENT PER 40 26 34% % 20 13% 6% 0 LOCAL UE EXTRA UE Supply chain 114 |
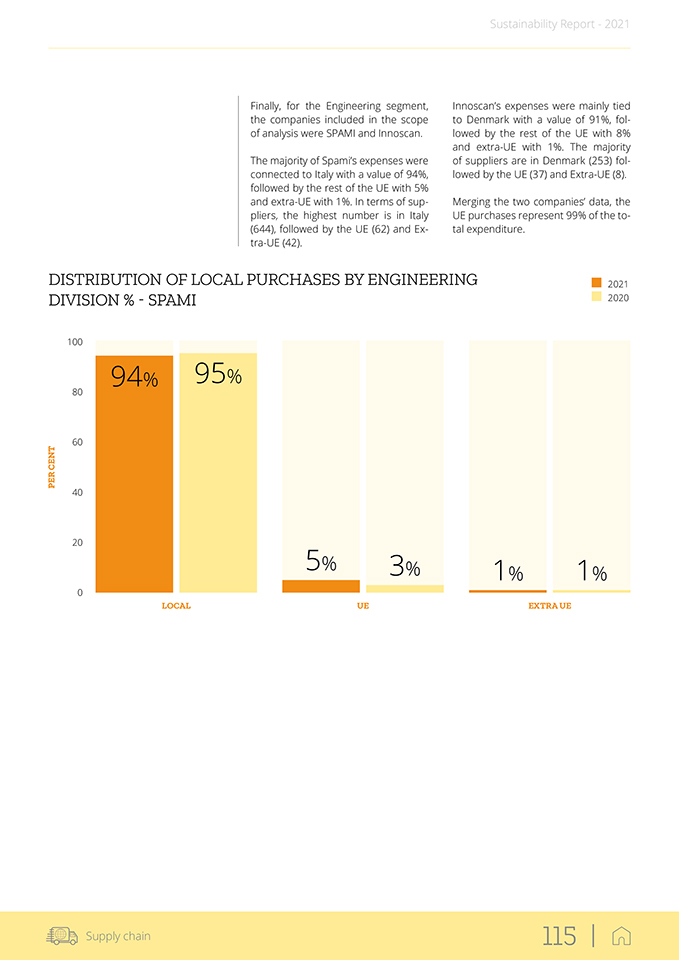
Sustainability Report—2021 Finally, for the Engineering segment, the companies included in the scope of analysis were SPAMI and Innoscan. The majority of Spami’s expenses were connected to Italy with a value of 94%, followed by the rest of the UE with 5% and extra-UE with 1%. In terms of suppliers, the highest number is in Italy (644), followed by the UE (62) and Extra-UE (42). Innoscan’s expenses were mainly tied to Denmark with a value of 91%, followed by the rest of the UE with 8% and extra-UE with 1%. The majority of suppliers are in Denmark (253) followed by the UE (37) and Extra-UE (8). Merging the two companies’ data, the UE purchases represent 99% of the total expenditure. DISTRIBUTION OF LOCAL PURCHASES BY ENGINEERING 2021 DIVISION %—SPAMI 2020 100 94% 95% 80 60 CENT PER 40 20 5% 3% 1% 1% 0 LOCAL UE EXTRA UE Supply chain 115 |
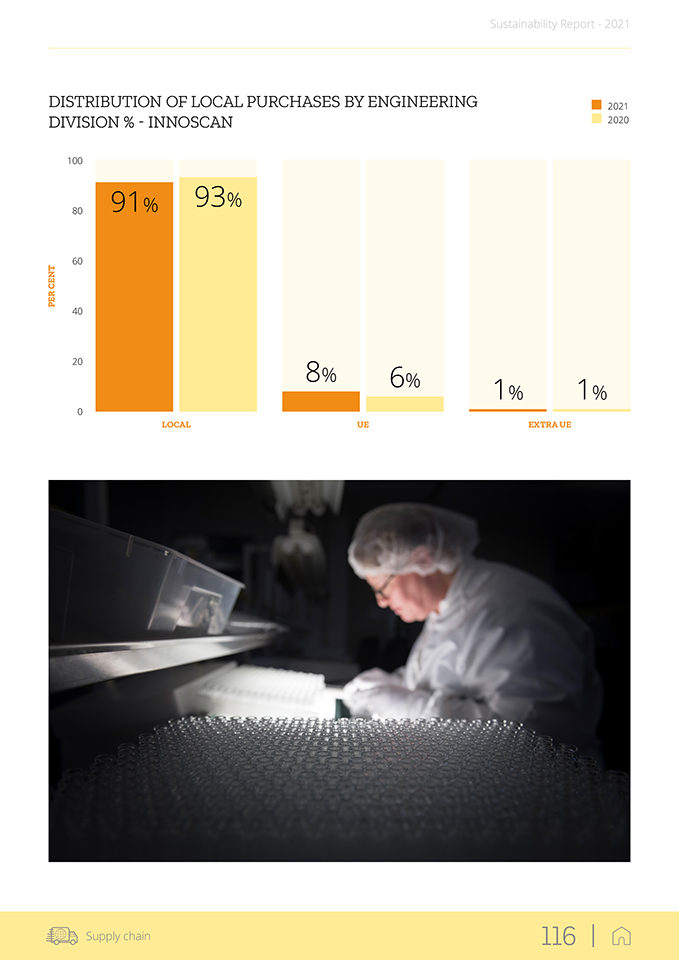
Sustainability Report—2021 DISTRIBUTION OF LOCAL PURCHASES BY ENGINEERING 2021 DIVISION %—INNOSCAN 2020 100 91% 93% 80 60 CENT PER 40 20 8% 6% 1% 1% 0 LOCAL UE EXTRA UE Supply chain 116 |

Attention to the environment 7

Sustainability Report—2021 Stevanato Group relentlessly seeks to reduce the environmental impact of all its processes and products. Accordingly, the Group is committed to the ongoing pursuit of the latest sustainable solutions in its field. This commitment is reflected by the Group’s EHS policy, which strives to find the right balance between environmental impact and economic growth. Environmentally-friendly measures taken by SG include: optimization of energy use and efficiency; optimization of natural resource consumption; reduction of waste and emissions using the best available technologies; and the recovery of materials from its production activities. Environment 7.1 Stevanato Group’s commitment to the environment Furthermore, Stevanato is committed to complying with the applicable environmental legislation and regulations, adopting the necessary precautions and provisions for the prevention of environmental pollution, promoting awareness and responsibility among all employees, providing appropriate information and training programs, and making its environmental policy available to the public. Stevanato is committed to carrying out its activities responsibly and to protecting the environment as outlined in its EHS policy, which reflects the principles and values set out in the company’s Code of Ethics. In line with these policies, the Group has identified the following lines of strategic action: • Minimization of environmental impact through constant monitoring and mapping of energy consumption; promotion of policies, initiatives and actions designed to optimize energy efficiency through “green” technologies; and procuring energy from renewable sources. • Waste and Air emissions reduction through monitoring, preventing and reducing emissions of main pollutants into the atmosphere, mainly related to the company’s production processes; promoting waste management awareness, with particular reference to hazardous waste, in compliance with regulations; promoting and implementing methods and practices for the reduction of waste produced; focusing on recycling, recovery and reuse of production waste as part of corporate Circular Economy innovation program. 118 |

SHARING ENVIRONMENTAL RESULTS WITH STAKEHOLDERS As part of its commitment to ethics, the Group actively involves its stakeholders in the continuous improvement of processes to bring responsible development to the communities where it operates. The environmental performance has a direct and indirect impact on human health and on the environment. SG believes that promoting employee engagement and individual responsibility for environmental actions is an important part of its success. Therefore, it encourages employees to take an active part in the process by sharing information on environmental and sustainability issues. Stevanato communicates information about waste production, natural gas and energy consumption on its website for all stakeholders and provides an overview of data related to the environment on the intranet for employees. Sustainability Report—2021 Stevanato Group is compliant with ISO 14001 Environmental Management System in seven sites. The synergistic use of these ISO and specific GRI Standards regarding the environment makes it easier for the Group to lessen its environmental impact and creates a sustainability model that is measurable, promotable and comparable. In order to constantly monitor its environmental footprint, SG uses a centralized management system that identifies potential risks in advance, enacting preventive measures and attentive supervision. The environmental data presented refers to all the Group’s production sites. It does not include SG’s commercial companies, which are deemed immaterial, given the small number of employees and their non-engagement in production activities. Any other exceptions or limitations to the scope are specified below in each table. The Group is committed to exceeding customer expectations in its respect for the environment and sustainability. Particularly, the company defined an EHS Policy in 2018 and updated in 2021, aiming to ensure the health and safety of its employees, customers, end-users, suppliers, partners and visitors, as well as protect the environment. Stevanato Group operates its worldwide business under the principles of sustainability, putting health, safety and environmental management at the heart of its business processes. In particular, the Group implements sustainability measures in the following areas: • energy efficiency and renewable energy (e.g., trigeneration system, heat recovery, solar energy); • water management (ref. water recovery for second use / closed loop); • waste management (mainly reuse, recycling and Waste-To-Value practices for glass and plastics scraps). Stevanato has identified the possible environmental impact areas of its business thanks to the materiality analysis (see chapter 2.1 Approach to sustainability), which include energy consumption, GHG emissions, water management and waste management. The Group regularly monitors and manages these impact areas to ensure compliance with the ruling legal requirements of the countries where it operates. Finally, Stevanato Group monitors and ensures active compliance with laws, ethical standards, and national and international norms. As a confirmation of these commitments, in 2021 the Group received no significant fines or non-monetary sanctions for non-compliance with environmental laws and/or regulations. Environment 119 |

Electricity and natural gas remain the main energy sources used in production processes. Energy consumption is one of the main indicators of environmental impact stemming from the Group’s production facilities. The direct and indirect emissions of Italian and foreign production sites are monitored centrally via the regular analysis of primary energy consumption, such as natural gas for heating and electricity for buildings. Stevanato Group has introduced a number of initiatives focused on mitigating energy consumption. In 2021, the following activities were carried out in conjunction with foreign production sites, such as: Sustainability Report—2021 For several years, Stevanato Group has implemented careful and responsible management of its energy consumption. For companies in the Glass (DCS, Drug Containment Solutions) business, the main energy sources are natural gas and electricity, whereas for the Plastics (DDS, Drug Delivery Systems; IVD, In Vitro Diagnostic) Solutions and Engineering segment, the main energy vector is electricity. Additional consumption derives from diesel fuel both for heating in Ompi of Brazil and, in Ompi North America, from generator systems and transportation of the company’s logistic vehicles. Diesel fuel used for company transportation includes the Balda facilities in the USA and all Italian plants. To advance sustainable mobility, the Group began upgrading its company fleet to hybrid vehicles in 2021. Environment 7.2 Energy Consumption and GHG Emissions • Development of Energy Management services to monitor, control and optimize the efficiency of its electric utility grid at the DCS plant in Piombino Dese and in Germany at Balda Medical. • Use of alternative energy production systems: since 2020, the Group has utilized a leased trigeneration plant (simultaneous generation of electri- city and heating and cooling) fueled by natural gas at its headquarters in Piombino Dese (Italy), significantly reducing emissions. • Improvements in lighting systems: Stevanato Group has reduced ener- gy inefficiency with a notable in- vestment in LED technologies. This improvement has also significantly increased the average lifespan of its lighting systems. • Replacement of air systems: over the last three years, the Group has replaced compressed air pro- duction systems with higher efficien- cy systems in its plants. Stevanato Group monitors key parameters like pressure, flow, power and dew point and, once collected, analyzes the data to verify any issues. 120 |
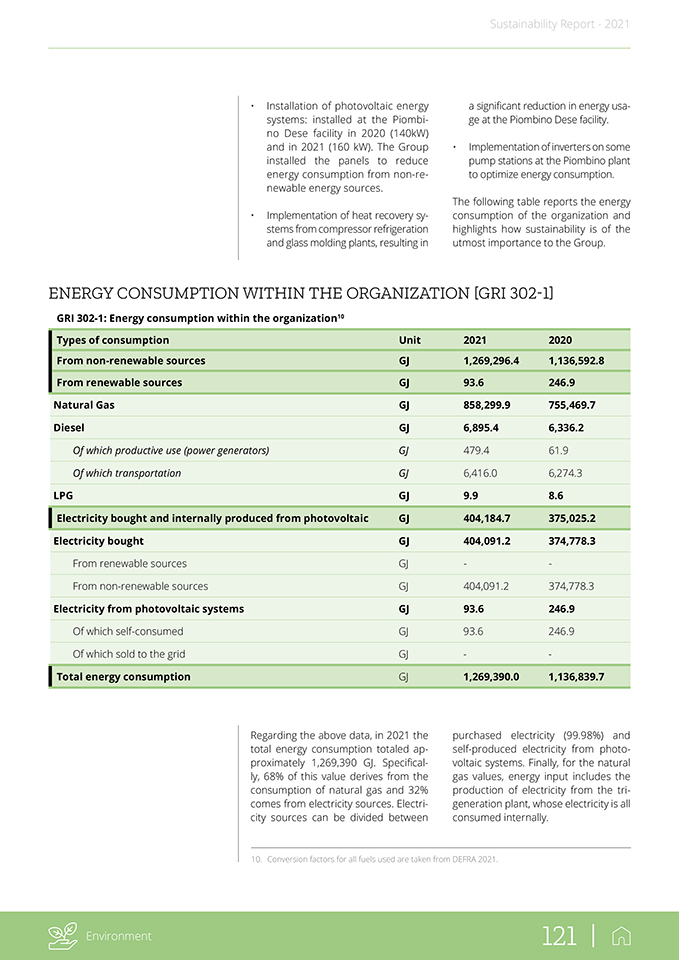
GRI 302-1: Energy consumption within the organization10 Types of consumption Unit 2021 2020 From non-renewable sources GJ 1,269,296.4 1,136,592.8 From renewable sources GJ 93.6 246.9 Natural Gas GJ 858,299.9 755,469.7 Diesel GJ 6,895.4 6,336.2 Of which productive use (power generators) GJ 479.4 61.9 Of which transportation GJ 6,416.0 6,274.3 LPG GJ 9.9 8.6 Electricity bought and internally produced from photovoltaic GJ 404,184.7 375,025.2 Electricity bought GJ 404,091.2 374,778.3 From renewable sources GJ — From non-renewable sources GJ 404,091.2 374,778.3 Electricity from photovoltaic systems GJ 93.6 246.9 Of which self-consumed GJ 93.6 246.9 Of which sold to the grid GJ — Total energy consumption GJ 1,269,390.0 1,136,839.7 Sustainability Report—2021 • Installation of photovoltaic energy systems: installed at the Piombi-no Dese facility in 2020 (140kW) and in 2021 (160 kW). The Group installed the panels to reduce energy consumption from non-renewable energy sources. • Implementation of heat recovery systems from compressor refrigeration and glass molding plants, resulting in a significant reduction in energy usage at the Piombino Dese facility. • Implementation of inverters on some pump stations at the Piombino plant to optimize energy consumption. The following table reports the energy consumption of the organization and highlights how sustainability is of the utmost importance to the Group. ENERGY CONSUMPTION WITHIN THE ORGANIZATION [GRI 302-1] Regarding the above data, in 2021 the total energy consumption totaled approximately 1,269,390 GJ. Specifically, 68% of this value derives from the consumption of natural gas and 32% comes from electricity sources. Electricity sources can be divided between purchased electricity (99.98%) and self-produced electricity from photovoltaic systems. Finally, for the natural gas values, energy input includes the production of electricity from the tri-generation plant, whose electricity is all consumed internally. 10. Conversion factors for all fuels used are taken from DEFRA 2021. Environment 121 |

• Scope 1: direct emissions associated with sources owned or controlled by the Group, used as fuels for heating and operational needs ; • Scope 2: indirect emissions deriving from the consumption of electricity purchased by Stevanato. Sustainability Report—2021 An increase of 12% in energy consumption in 2021 compared with 2020 is mostly due to an increase in production volumes (+16%) in addition to the implementation of new production lines for EZ-fill® areas. There was a decrease in the self-production of electricity in 2021 due to the temporary downtime of the photovoltaic system. Below are the main types of emissions related to the above-mentioned energy sources. In particular, to report greenhouse gas emissions, Stevanato Group has joined the Greenhouse Gas (GHG) Protocol, which requires the distinction of emissions into categories or “Scope”: Specifically, in compliance with GRI Reporting Standards, emissions are calculated according to Location and Market methodologies, using appropriate emission factors. The Group places particular focus on GHG emissions with regards to compliance with local regulations. Specific interest is applied to air emission reductions as defined in the EHS Policy. Emissions are calculated by directly measuring the relevant energy source and the conversion into GHG in order to determine the value of the CO2 equivalent. Direct (Scope 1) GHG emissions include the CO2 emissions from the energy fuel consumption as reported in Disclosure GRI 302-1. Regarding direct emissions (Scope 1), Stevanato Group produced a total of 44,254 of tons of CO2e in 2021. The data provided considers CO2 emissions deriving mainly from the use of natural gas, diesel fuel and refrigerant gas. The following table shows the strong influence of natural gas in the direct greenhouse gas emissions (Scope 1), which accounts for 99% of the total value. The remaining percentage derives mainly from diesel fuel used for both heating and transportation, and refrigerant gas. The increase of 12% in Scope 1 emissions in 2021 compared with 2020 is mostly due to an increase in production volumes (+16%) in addition to diesel consumption in OMPI Do Brazil used for energy generators due to a shortage of electricity from its supplier. Environment 122 |
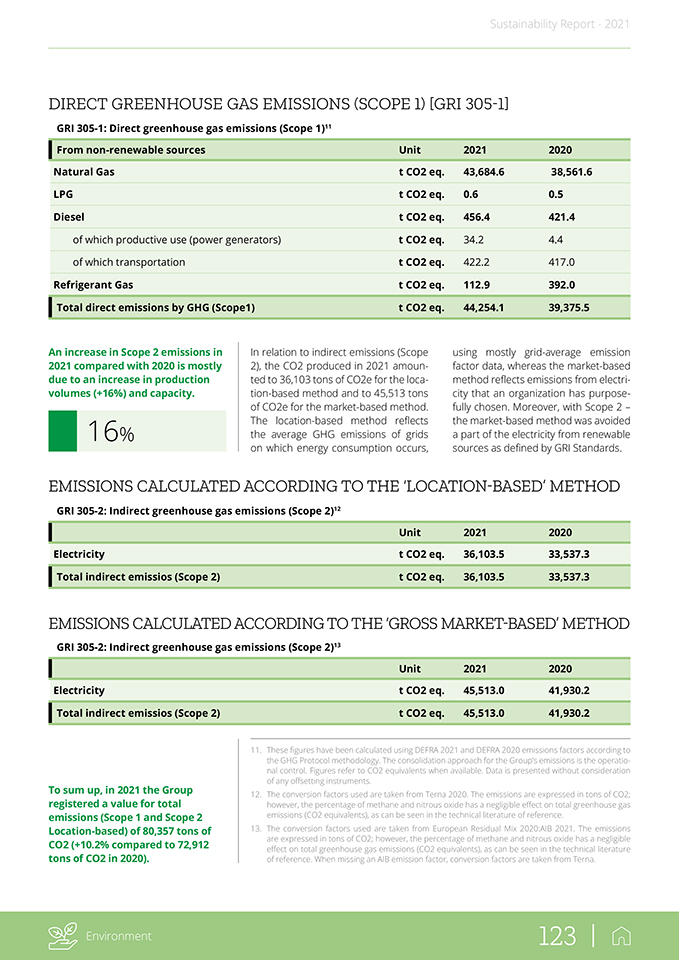
GRI 305-1: Direct greenhouse gas emissions (Scope 1)11 From non-renewable sources Unit 2021 2020 Natural Gas t CO2 eq. 43,684.6 38,561.6 LPG t CO2 eq. 0.6 0.5 Diesel t CO2 eq. 456.4 421.4 of which productive use (power generators) t CO2 eq. 34.2 4.4 of which transportation t CO2 eq. 422.2 417.0 Refrigerant Gas t CO2 eq. 112.9 392.0 Total direct emissions by GHG (Scope1) t CO2 eq. 44,254.1 39,375.5 Sustainability Report—2021 DIRECT GREENHOUSE GAS EMISSIONS (SCOPE 1) [GRI 305-1] An increase in Scope 2 emissions in In relation to indirect emissions (Scope using mostly grid-average emission 2021 compared with 2020 is mostly 2), the CO2 produced in 2021 amoun- factor data, whereas the market-based due to an increase in production ted to 36,103 tons of CO2e for the loca- method reflects emissions from electri-volumes (+16%) and capacity. tion-based method and to 45,513 tons city that an organization has purpose-of CO2e for the market-based method. fully chosen. Moreover, with Scope 2 –The location-based method reflects the market-based method was avoided 16% the average GHG emissions of grids a part of the electricity from renewable on which energy consumption occurs, sources as defined by GRI Standards. EMISSIONS CALCULATED ACCORDING TO THE ‘LOCATION-BASED’ METHOD GRI 305-2: Indirect greenhouse gas emissions (Scope 2)12 Unit 2021 2020 Electricity t CO2 eq. 36,103.5 33,537.3 Total indirect emissios (Scope 2) t CO2 eq. 36,103.5 33,537.3 EMISSIONS CALCULATED ACCORDING TO THE ‘GROSS MARKET-BASED’ METHOD GRI 305-2: Indirect greenhouse gas emissions (Scope 2)13 Unit 2021 2020 Electricity t CO2 eq. 45,513.0 41,930.2 Total indirect emissios (Scope 2) t CO2 eq. 45,513.0 41,930.2 To sum up, in 2021 the Group registered a value for total emissions (Scope 1 and Scope 2 Location-based) of 80,357 tons of CO2 (+10.2% compared to 72,912 tons of CO2 in 2020). 11. These figures have been calculated using DEFRA 2021 and DEFRA 2020 emissions factors according to the GHG Protocol methodology. The consolidation approach for the Group’s emissions is the operational control. Figures refer to CO2 equivalents when available. Data is presented without consideration of any offsetting instruments. 12. The conversion factors used are taken from Terna 2020. The emissions are expressed in tons of CO2; however, the percentage of methane and nitrous oxide has a negligible effect on total greenhouse gas emissions (CO2 equivalents), as can be seen in the technical literature of reference. 13. The conversion factors used are taken from European Residual Mix 2020:AIB 2021. The emissions are expressed in tons of CO2; however, the percentage of methane and nitrous oxide has a negligible effect on total greenhouse gas emissions (CO2 equivalents), as can be seen in the technical literature of reference. When missing an AIB emission factor, conversion factors are taken from Terna. Environment 123 |

Water management is a key aspect of Stevanato’s environmental approach and a significant topic noted by SG stakeholders in the materiality analysis in terms of: • Defining strategies to increase efficiency and reduce water use, especially for the Group’s production activities, including water reuse and recycling methods; • Implementing and monitoring effective measures for the correct management of industrial wa-stewater discharges, especially discharges containing dangerous substances, in full compliance with current regulations. Sustainability Report—2021 7.3 Water management The Drug Containment Solutions companies draw 74% of their water from local underground sources, with the remaining 26% coming from the public water supply. Water is used in the production process in the following operations: • as washer and sanitizer for semi-finished glass products for pharmaceutical companies; • as a carrier fluid in cooling systems; • cleaner for hygienic uses; • catering for company canteen. 124 |

Companies in the DDS, IVD and Engineering segments use water almost exclusively for hygiene, cleaning and catering purposes. The water used in these plants comes from public services. The companies related to Ompi, Medical Glass and Balda Medical are ISO 14001 certified and overall water usage is assessed according to the protocols dictated by the certification standards as part of environmental impact analyses. The data obtained is periodically reviewed for Group reporting and communication to local authorities, as well as for monitoring environmental performance. The significant amount of water withdrawn from public services and groundwater, which is discharged by the organization post-production, represents the main environmental impact. By progressively improving the quality of water discharged, promoting its reuse in the industrial environment, and reducing its withdrawal by optimizing processes, the organization can reduce its impact on the surrounding environment. On one hand, the quality of the water discharged by the Group’s plants, due to the processes carried out during normal operations, has little impact on external watercourses and treatment plants. On the other hand, the amount of water withdrawn by Stevanato and the quantity of its discharges can impact the ecosystem in numerous ways. Balda Medical, Germany The total amount of water withdrawal by public service (third parties) comes from La Weser, which is a river of Lower Saxony in north-west Germany. Sustainability Report—2021 Direct impacts on a catchment can have wider impacts on the quality of life in an area, including social and economic consequences for local communities. An important initiative started at the headquarters in February 2020 is the modification of the water distribution system in order to re-utilize the fluid coming from the semi-finished product sanitizing process (WFI – Water For Injection). After this initial process (WFI), the water is channeled for filtering and cooling so that it can then be reused. This new implementation at the Piombi-no Dese plant has helped reduce water withdrawal by 10,000 m3/year. After treatment, water coming from production processes is made suitable for irrigation/gardening at Ompi North America (4144 m3/year) and Ompi Do Brasil (3007 m3/year). Starting in 2020, water withdrawal at production sites has also been monitored to assess locally-based water stress. For the identification of water-stressed areas, the Water Resource Institute tool is utilized, providing a more comprehensive overview of the organization’s water use and water-related impacts, and possible measures to manage them. According to the Water Resource Institute Tool, the entities located in high and extremely high-water stressed areas are: Ompi North America, Mexico 12% of the total amount of water withdrawal is by public service (third parties) and comes from Río Bravo del Norte, which is one of the principal rivers (along with the Colorado River) in the south-western United States and northern Mexico. Balda Precision, Oceanside (CA) The total amount of water withdrawal by public service (third parties) comes from the Colorado River, Carlsbad’s desalination plant, the California Aqueduct system, and recycled water programs. Balda C. Brewer Ontario (CA) The total amounts of water withdrawal by public service (third parties) comes from Santa Ana River Lakes, which are located in the city of Anaheim, California. Environment 125 |
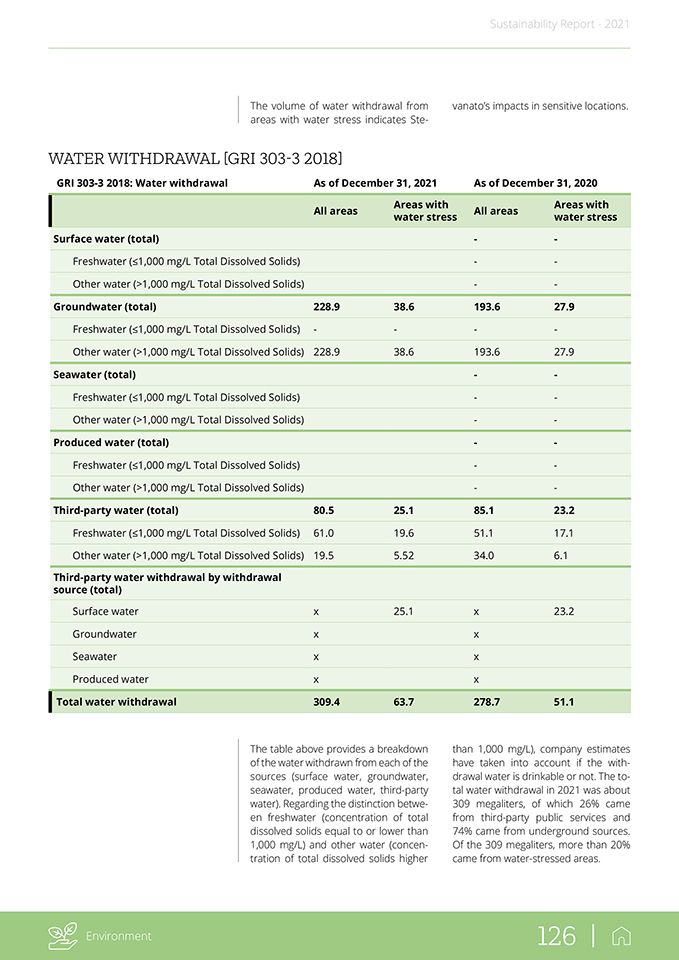
Companies in the DDS, IVD and Engineering segments use water almost exclusively for hygiene, cleaning and catering purposes. The water used in these plants comes from public services. The companies related to Ompi, Medical Glass and Balda Medical are ISO 14001 certified and overall water usage is assessed according to the protocols dictated by the certification standards as part of environmental impact analyses. The data obtained is periodically reviewed for Group reporting and communication to local authorities, as well as for monitoring environmental performance. The significant amount of water withdrawn from public services and groundwater, which is discharged by the organization post-production, represents the main environmental impact. By progressively improving the quality of water discharged, promoting its reuse in the industrial environment, and reducing its withdrawal by optimizing processes, the organization can reduce its impact on the surrounding environment. On one hand, the quality of the water discharged by the Group’s plants, due to the processes carried out during normal operations, has little impact on external watercourses and treatment plants. On the other hand, the amount of water withdrawn by Stevanato and the quantity of its discharges can impact the ecosystem in numerous ways. Balda Medical, Germany The total amount of water withdrawal by public service (third parties) comes from La Weser, which is a river of Lower Saxony in north-west Germany. Sustainability Report—2021 Direct impacts on a catchment can have wider impacts on the quality of life in an area, including social and economic consequences for local communities. An important initiative started at the headquarters in February 2020 is the modification of the water distribution system in order to re-utilize the fluid coming from the semi-finished product sanitizing process (WFI – Water For Injection). After this initial process (WFI), the water is channeled for filtering and cooling so that it can then be reused. This new implementation at the Piombi-no Dese plant has helped reduce water withdrawal by 10,000 m3/year. After treatment, water coming from production processes is made suitable for irrigation/gardening at Ompi North America (4144 m3/year) and Ompi Do Brasil (3007 m3/year). Starting in 2020, water withdrawal at production sites has also been monitored to assess locally-based water stress. For the identification of water-stressed areas, the Water Resource Institute tool is utilized, providing a more comprehensive overview of the organization’s water use and water-related impacts, and possible measures to manage them. According to the Water Resource Institute Tool, the entities located in high and extremely high-water stressed areas are: Ompi North America, Mexico 12% of the total amount of water withdrawal is by public service (third parties) and comes from Río Bravo del Norte, which is one of the principal rivers (along with the Colorado River) in the south-western United States and northern Mexico. Balda Precision, Oceanside (CA) The total amount of water withdrawal by public service (third parties) comes from the Colorado River, Carlsbad’s desalination plant, the California Aqueduct system, and recycled water programs. Balda C. Brewer Ontario (CA) The total amounts of water withdrawal by public service (third parties) comes from Santa Ana River Lakes, which are located in the city of Anaheim, California. Environment 125 |
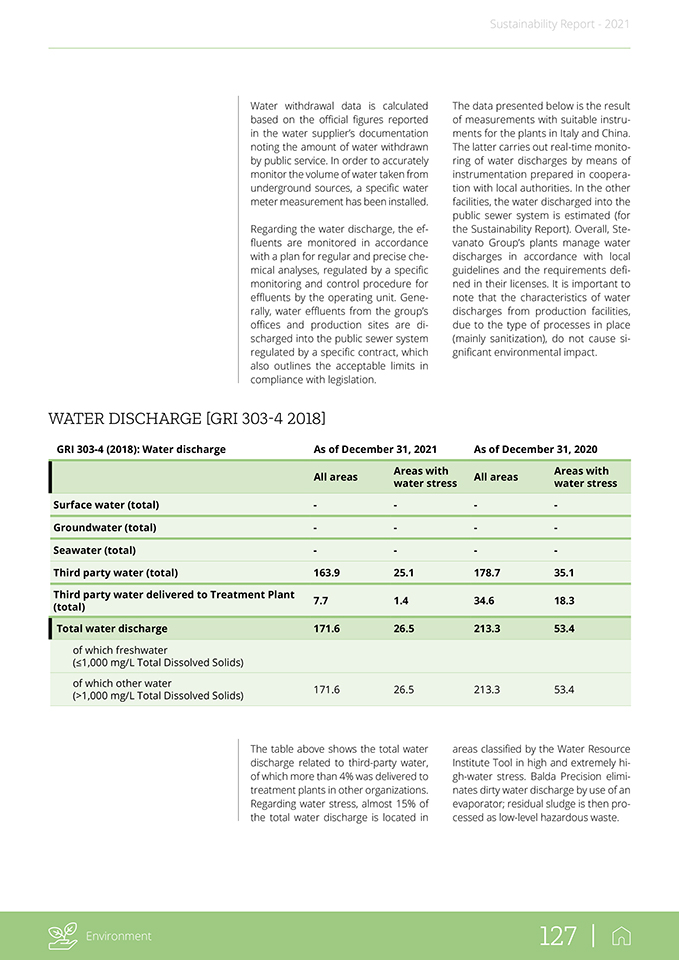
Water withdrawal data is calculated based on the official figures reported in the water supplier’s documentation noting the amount of water withdrawn by public service. In order to accurately monitor the volume of water taken from underground sources, a specific water meter measurement has been installed. Regarding the water discharge, the effluents are monitored in accordance with a plan for regular and precise chemical analyses, regulated by a specific monitoring and control procedure for effluents by the operating unit. Generally, water effluents from the group’s offices and production sites are discharged into the public sewer system regulated by a specific contract, which also outlines the acceptable limits in compliance with legislation. Sustainability Report—2021 The data presented below is the result of measurements with suitable instruments for the plants in Italy and China. The latter carries out real-time monitoring of water discharges by means of instrumentation prepared in cooperation with local authorities. In the other facilities, the water discharged into the public sewer system is estimated (for the Sustainability Report). Overall, Ste-vanato Group’s plants manage water discharges in accordance with local guidelines and the requirements defined in their licenses. It is important to note that the characteristics of water discharges from production facilities, due to the type of processes in place (mainly sanitization), do not cause significant environmental impact. WATER DISCHARGE [GRI 303-4 2018] GRI 303-4 (2018): Water discharge As of December 31, 2021 As of December 31, 2020 Areas with Areas with All areas All areas water stress water stress Surface water (total) — —Groundwater (total) — —Seawater (total) — —Third party water (total) 163.9 25.1 178.7 35.1 Third party water delivered to Treatment Plant 7.7 1.4 34.6 18.3 (total) Total water discharge 171.6 26.5 213.3 53.4 of which freshwater ( 1,000 mg/L Total Dissolved Solids) of which other water 171.6 26.5 213.3 53.4 (>1,000 mg/L Total Dissolved Solids) The table above shows the total water discharge related to third-party water, of which more than 4% was delivered to treatment plants in other organizations. Regarding water stress, almost 15% of the total water discharge is located in areas classified by the Water Resource Institute Tool in high and extremely high-water stress. Balda Precision eliminates dirty water discharge by use of an evaporator; residual sludge is then processed as low-level hazardous waste. Environment 127 |

Sustainability Report—2021 WATER INITIATIVES IN MEXICO Ompi North America, Mexico, has designed an initiative called Brackish that consists of the desalination of underground water to be used in the production process. Once the facility has used the water, it proceeds to discharge it in order to replenish aquifers and rivers with fresh water. In exchange for the discharge of desalinated water, the local governmental authority offers a discount to Ompi on its water consumption bill. The project is in line with SG’s mission to raise awareness and encourage responsibility for the environment, which, if managed efficiently, also generates significant savings for the company. The Brackish project has saved more than 2,000 € and 4 megaliters of water withdrawals a year. Environment 128 |

Sustainability Report—2021 Stevanato Group pays special attention to waste production, respecting all the mandatory regulations in every country where it operates and always striving to minimize the total amount of waste. As defined in the EHS Policy, specific focus is applied to waste reduction, implementing the best available techniques and reporting results to stakeholders. 7.4 Waste management The waste produced by the Group’s companies derives from the production processes and comes mainly from warehousing (packaging materials), production (production and quality waste) and ancillary activities such as maintenance and office work. The plants dedicated to the production of glass primary packaging generate several types of waste, depending on the nature of manufacturing operations. In this area, Stevanato stresses the importance of circularity in its production process as per the corporate Circular Economy innovation program. The circular economy is a set of principles that aims to turn products at the end of their service life into resources for others, closing loops in industrial ecosystems, minimizing waste and following sustainable practices. The circular economy is a strategic lever in Environment 129 |

tackling resource scarcity, global warming and waste management, and is prominently reflected in the Stevanato EHS policy. In this context, Stevana-to Group implements Waste to Value practices for glass and plastics scraps, giving new purpose to material that would otherwise become trash. In addition, Balda recycles the in-process trays in a circular loop. Among its initiatives, the Group endeavors to reduce single-use plastics by providing simple alternatives. In several plants, Stevanato has already eliminated PET plastic bottle consumption, replacing 500 ml bottles with coolers and 20-liter jugs in the office areas. In addition, Stevanato is also committed to reducing food waste in company canteens. In Brazil it has started a zero-food waste initiative, whereas in Mexico, it has implemented a meticulous system to differentiate and properly manage food waste, following the local recycling regulations. Balda Precision recycles all of its metal scrap and filters as well as machining oil to keep hazardous waste to an absolute minimum. The waste management process involves collecting and storing waste in defined areas and specific containers according to the type of waste. The volumes managed are controlled by measuring the masses delivered to the disposal plants; the relevant data is stored in a specific database for each plant and shared at group level so that it can be monitored by the central EHS department. Waste for disposal is entrusted to third parties who operate in compliance with the relevant contractual or legal regulations. Data on waste production is provided regularly in order to assess performance and ensure regular reporting to public bodies. The data below is the result of measurements carried out with appropriate instruments at waste disposal companies. In fact, all waste generated at Stevanato Group plants is stored temporarily at the production sites and transported by Sustainability Report—2021 authorized suppliers to storage facilities for further management and disposal. At this moment, the company does not directly recycle the production waste back into the value chain at its sites, mainly because of the pharma requirements. However, SG has started to investigate such opportunities as part of its corporate Circular Economy innovation program. All Stevanato plants are committed to implementing material reuse policies such as recyclable containers for the transport of raw materials and semi-finished products. A review of processes is also carried out to optimize the raw material input and the reduction of waste materials. Furthermore, all the waste from facilities is collected, differentiated and, wherever possible, recycled (plastic, brass chips, aluminum, stainless steel). The waste generated by Stevanato Group consists mainly of: • Recyclable waste: glass, cardboard, plastic, wooden pallets, exhaust oil, colors and solvents, laboratory chemicals, batteries, accumulators, iron and fluorescent tubes; • Not-recyclable waste: Freon exhaust, garbage, toners, packaging containing residues of dangerous substances, absorbents, oil rags. Most of the waste listed above comes from activities related to Ste-vanato Group organizations in the Glass (DCS) division, which accounts for 91% of the total waste produced, the Plastics (DDS, IVD) division, which accounts for 7%, and the Engineering division (2%). Taking all products produced annually into account, Stevana-to Group produces more than 10,400 tons of industrial process waste. Hazardous waste accounts for 7% of the total volume of waste produced. The percentage of waste recycling and reuse in the different divisions is remarkable, varying from 65% in the Glass (DCS) division to 79% in the Plastics (DDS, IVD) division, and 79% in the Engineering division. Environment 130 |
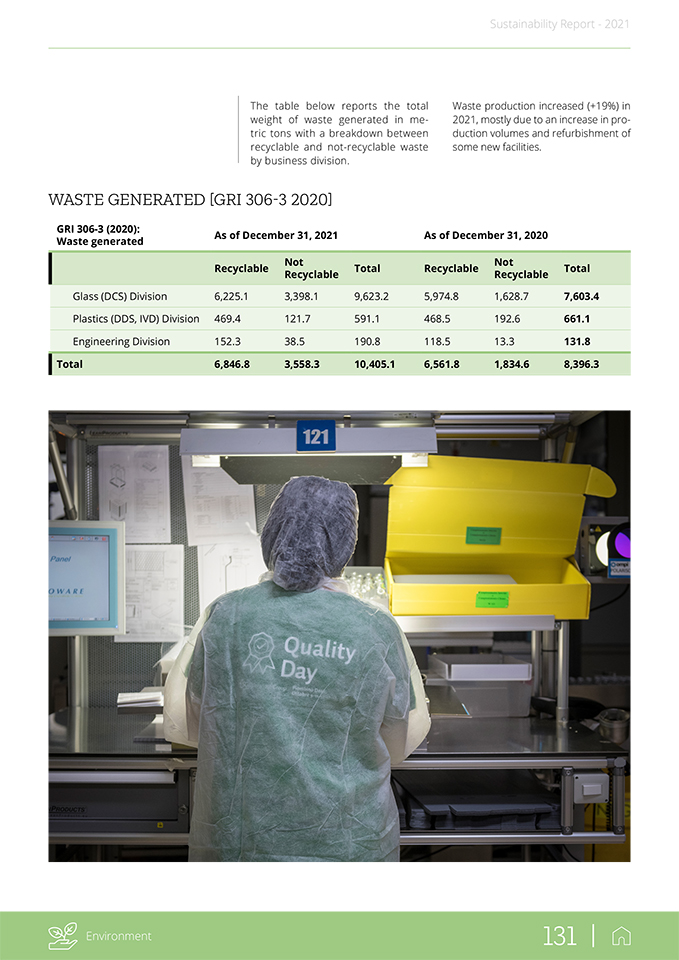
Sustainability Report—2021 The table below reports the total weight of waste generated in metric tons with a breakdown between recyclable and not-recyclable waste by business division. Waste production increased (+19%) in 2021, mostly due to an increase in production volumes and refurbishment of some new facilities. WASTE GENERATED [GRI 306-3 2020] GRI 306-3 (2020): As of December 31, 2021 As of December 31, 2020 Waste generated Not Not Recyclable Total Recyclable Total Recyclable Recyclable Glass (DCS) Division 6,225.1 3,398.1 9,623.2 5,974.8 1,628.7 7,603.4 Plastics (DDS, IVD) Division 469.4 121.7 591.1 468.5 192.6 661.1 Engineering Division 152.3 38.5 190.8 118.5 13.3 131.8 Total 6,846.8 3,558.3 10,405.1 6,561.8 1,834.6 8,396.3 Environment 131 |
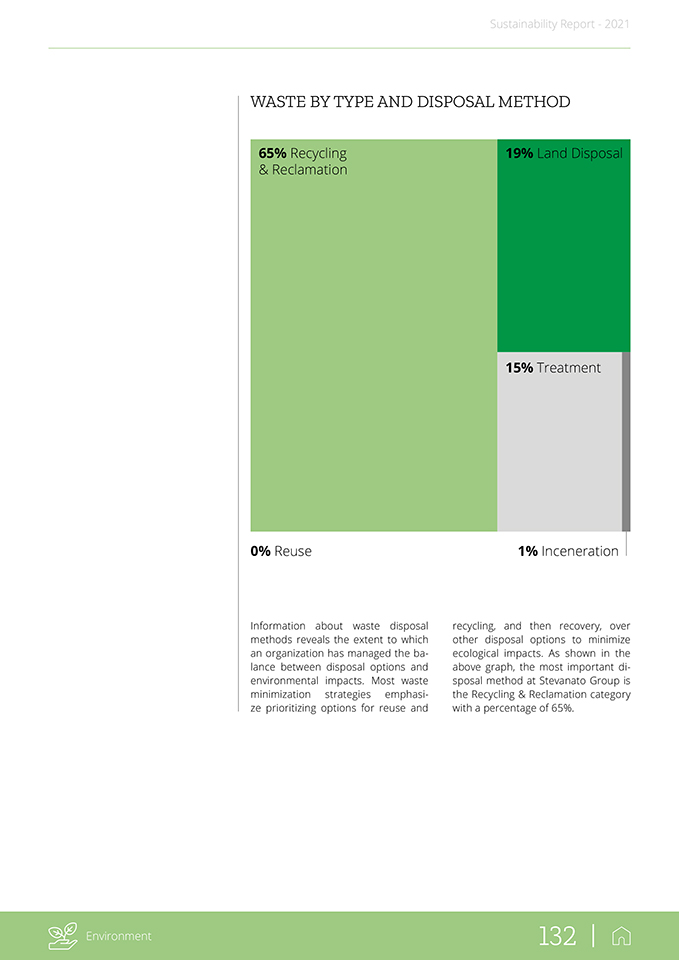
Sustainability Report—2021 WASTE BY TYPE AND DISPOSAL METHOD 65% Recycling & Reclamation 0% Reuse Information about waste disposal methods reveals the extent to which an organization has managed the balance between disposal options and environmental impacts. Most waste minimization strategies emphasize prioritizing options for reuse and 19% Land Disposal 15% Treatment 1% Inceneration recycling, and then recovery, over other disposal options to minimize ecological impacts. As shown in the above graph, the most important disposal method at Stevanato Group is the Recycling & Reclamation category with a percentage of 65%. Environment 132 |

The link with the territory 8

8.1 Local communities engagement Sustainability Report—2021 Stevanato Group has always been closely connected to the land and the communities where it operates. In Italy, where it has been established for 70 years, Stevanato Group has shown its commitment to the local territory over time, reconciling its industrial development with an improvement in the overall quality of life of people. International developments are much more recent, as such, the link with communities is being steadily built as the Group’s companies grow, as demonstrated by the charitable donations made in China and Denmark over the last few years. From this ongoing and meaningful commitment to local community, and a desire to fund lasting projects with greater social impact, the decision to establish the Stevanato Foundation was born. The Foundation was established on October 26, 2017, and as a legal entity by Decree of the Ve-neto Region as of January 16, 2018. The founders and members of the Board of Directors are Sergio Stevana-to (Chairman), Franco Stevanato and Marco Stevanato. The Stevanato family provided the initial assets while the Italian companies contribute annually to support the Foundation’s mission. The Foundation pursues the aims of social solidarity, philanthropy and charity exclusively, operating in the fields of social and social-health care, educa- tion and training, and scientific research. In particular, it focuses on supporting children and young people with serious illnesses, disabilities, or other issues that may affect their health and growth, as well as their families. It does so by promoting educational, recreational and sports activities for youth, fostering social inclusion, encouraging the dissemination of civic values, supporting initiatives that have an immediately beneficial effect on society, and backing medical research projects that study illnesses and diseases. The interventions of the Stevanato Foundation, in both assets and funds, were distributed over a two-year period to the groups indicated in the following graphs: Territory 134 |
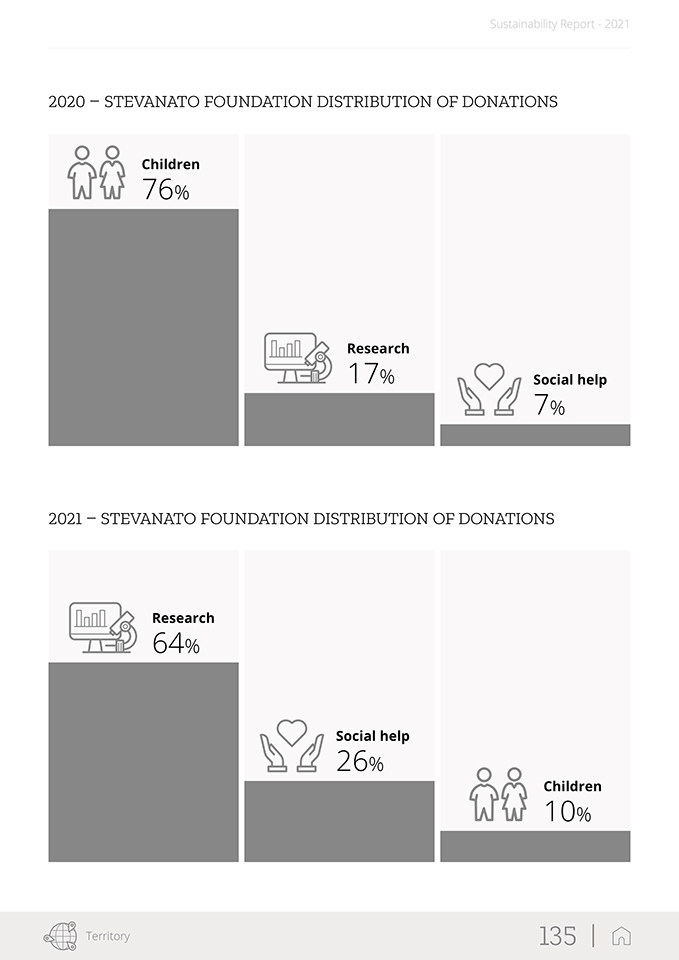
Sustainability Report—2021 2020 – STEVANATO FOUNDATION DISTRIBUTION OF DONATIONS Children 76% Research 17 % Social help 7% 2021 – STEVANATO FOUNDATION DISTRIBUTION OF DONATIONS Research 64% Social 26 help % Children 10% Territory 135 |

Sustainability Report—2021 The main projects and initiatives sup- establishment are reported below: ported by the Foundation since its Since 2018, the Stevanato Foundation has taken over support for V.I.M.M., a pillar of excellence at the international level for research in the field of cell and molecular biology, from Stevanato Group. In particular, the Foundation supports research projects on childhood diseases. Continuing the collaboration started by Stevanato Group in 2018, the Stevanato Foundation launched a multi-year project called “The 3.0 classroom” which introduces innovative teaching methods and new technologies so students can reach their full potential. The students become the main protagonists of the learning process and develop their creativity by solving problems and designing projects with others. The ULSS6 at Villa del Conte in Italy’s Veneto region has been restructured to begin caring for autistic children. The Stevanato Foundation contributed to the renovation of the facility. The new center, called “Rio Bio”, will engage a team of specialists to follow 162 cases of autistic children up to the age of 18. Thanks to the structural renovation, the existing spaces have been converted into health care areas, laboratories and spaces for therapeutic and group activities. The Foundation donated a car to the Elderly Movement Center of Piombino Dese, making it possible to activate a service for the home delivery of meals for elderly people who are not self-sufficient. The meals are cooked by a local cooperative in collaboration with the Foundation and the local administration. The Foundation contributed to the renovation of the outdoor playground area at the nursery in Piombino Dese, creating safe accessibility conditions for children with disabilities. Project ROP: project designed to provide the Mestre’s Hospital with a RetCam for early diagnosis and treatment of retinopathy in premature infants. The main objectives were: 1. “In house” diagnosis at Mestre’s Hospital, reducing children’s suffering and the inconvenience of travelling to other hospitals for their families; 2. Service for other hospitals in the district; 3. Enhancement of telemedicine (online diagnosis and consultation); 4. Enabling “in house” surgical treatment with increased specialist skills; 5. Support for diagnostics of other pathologies other than retinopathy. Territory 136 |

70TH ANNIVERSARY OF STEVANATO GROUP In 2019, Stevanato Group reached a major milestone, celebrating its 70th anniversary. Both customers and employees were invited to the headquarters in Piombino Dese and production plants around the world with the aim of thanking stakeholders for their support throughout the Group’s history and recognizing their role in the company’s growth. Stevanato opened its doors to the families of employees through “open factory” visits and hosted a “Family Day” featuring fun activities and thematic workshops for adults and children in our plants all over the world. Sustainability Report—2021 In addition to these initiatives, the Foundation has contributed to the well-being of its local communities through several non-profit donations which helped fund: a minibus for disabled people, wheelchair youth basketball and the Giovanni Stevanato trophy, and a machine that monitors vital stats (blood pressure, heart rate, saturation, electrocardiogram) for ambulances through the Italian Red Cross. In 2019, a collaboration between the Foundation, the municipality of Piombino Dese and its nurseries, was initiated to assist families in difficulty, help with payments of unpaid fees, and facilitate the contact between families and social services in cases of special need. Finally, in 2020, during the most critical phases of the Covid-19 pandemic emergency, Stevanato Group provided much-needed supplies and economic support to health and hospital facilities in the provinces of Venice, Padua and Treviso. Territory 137 |
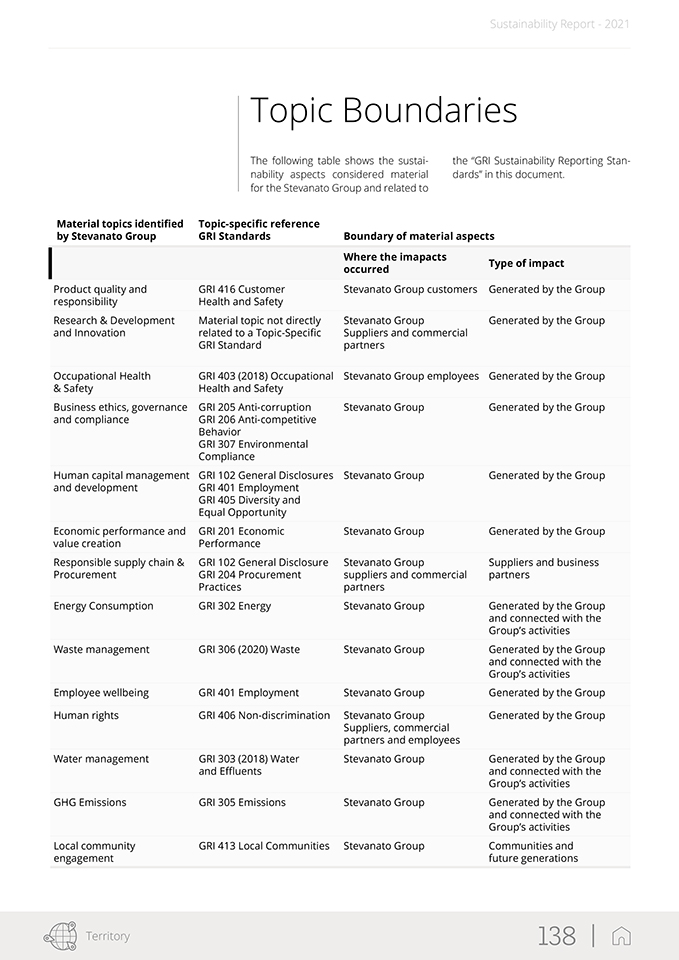
Product quality and GRI 416 Customer Stevanato Group customers Generated by the Group responsibility Health and Safety Research & Development Material topic not directly Stevanato Group Generated by the Group and Innovation related to a Topic-Specific Suppliers and commercial GRI Standard partners Occupational Health GRI 403 (2018) Occupational Stevanato Group employees Generated by the Group & Safety Health and Safety Business ethics, governance GRI 205 Anti-corruption Stevanato Group Generated by the Group and compliance GRI 206 Anti-competitive Behavior GRI 307 Environmental Compliance Human capital management GRI 102 General Disclosures Stevanato Group Generated by the Group and development GRI 401 Employment GRI 405 Diversity and Equal Opportunity Economic performance and GRI 201 Economic Stevanato Group Generated by the Group value creation Performance Responsible supply chain & GRI 102 General Disclosure Stevanato Group Suppliers and business Procurement GRI 204 Procurement suppliers and commercial partners Practices partners Energy Consumption GRI 302 Energy Stevanato Group Generated by the Group and connected with the Group’s activities Waste management GRI 306 (2020) Waste Stevanato Group Generated by the Group and connected with the Group’s activities Employee wellbeing GRI 401 Employment Stevanato Group Generated by the Group Human rights GRI 406 Non-discrimination Stevanato Group Generated by the Group Suppliers, commercial partners and employees Water management GRI 303 (2018) Water Stevanato Group Generated by the Group and Effluents and connected with the Group’s activities GHG Emissions GRI 305 Emissions Stevanato Group Generated by the Group and connected with the Group’s activities Local community GRI 413 Local Communities Stevanato Group Communities and engagement future generations Sustainability Report—2021 Topic Boundaries The following table shows the sustai- the “GRI Sustainability Reporting Stan-nability aspects considered material dards” in this document. for the Stevanato Group and related to Material topics identified Topic-specific reference by Stevanato Group GRI Standards Boundary of material aspects Where the imapacts Type of impact occurred Territory 138 |
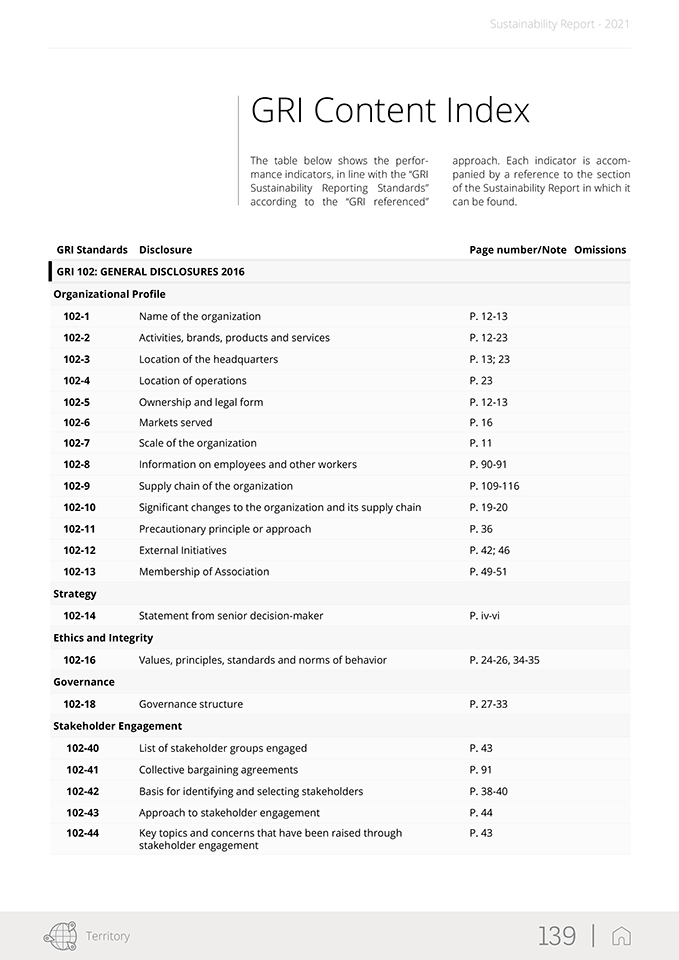
GRI Standards Disclosure Page number/Note Omissions GRI 102: GENERAL DISCLOSURES 2016 Organizational Profile 102-1 Name of the organization P. 12-13 102-2 Activities, brands, products and services P. 12-23 102-3 Location of the headquarters P. 13; 23 102-4 Location of operations P. 23 102-5 Ownership and legal form P. 12-13 102-6 Markets served P. 16 102-7 Scale of the organization P. 11 102-8 Information on employees and other workers P. 90-91 102-9 Supply chain of the organization P. 109-116 102-10 Significant changes to the organization and its supply chain P. 19-20 102-11 Precautionary principle or approach P. 36 102-12 External Initiatives P. 42; 46 102-13 Membership of Association P. 49-51 Strategy 102-14 Statement from senior decision-maker P. iv-vi Ethics and Integrity 102-16 Values, principles, standards and norms of behavior P. 24-26, 34-35 Governance 102-18 Governance structure P. 27-33 Stakeholder Engagement 102-40 List of stakeholder groups engaged P. 43 102-41 Collective bargaining agreements P. 91 102-42 Basis for identifying and selecting stakeholders P. 38-40 102-43 Approach to stakeholder engagement P. 44 102-44 Key topics and concerns that have been raised through P. 43 stakeholder engagement Sustainability Report—2021 GRI Content Index The table below shows the performance indicators, in line with the “GRI Sustainability Reporting Standards” according to the “GRI referenced” approach. Each indicator is accompanied by a reference to the section of the Sustainability Report in which it can be found. Territory 139 |
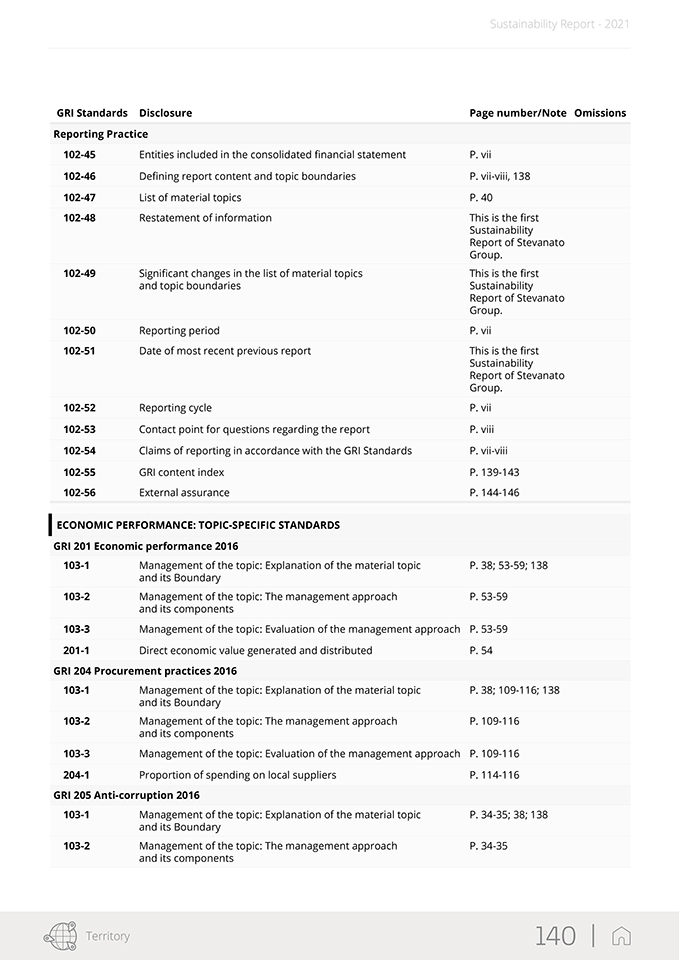
Sustainability Report—2021 GRI Standards Disclosure Page number/Note Omissions Reporting Practice 102-45 Entities included in the consolidated financial statement P. vii 102-46 Defining report content and topic boundaries P. vii-viii, 138 102-47 List of material topics P. 40 102-48 Restatement of information This is the first Sustainability Report of Stevanato Group. 102-49 Significant changes in the list of material topics This is the first and topic boundaries Sustainability Report of Stevanato Group. 102-50 Reporting period P. vii 102-51 Date of most recent previous report This is the first Sustainability Report of Stevanato Group. 102-52 Reporting cycle P. vii 102-53 Contact point for questions regarding the report P. viii 102-54 Claims of reporting in accordance with the GRI Standards P. vii-viii 102-55 GRI content index P. 139-143 102-56 External assurance P. 144-146 ECONOMIC PERFORMANCE: TOPIC-SPECIFIC STANDARDS GRI 201 Economic performance 2016 103-1 Management of the topic: Explanation of the material topic P. 38; 53-59; 138 and its Boundary 103-2 Management of the topic: The management approach P. 53-59 and its components 103-3 Management of the topic: Evaluation of the management approach P. 53-59 201-1 Direct economic value generated and distributed P. 54 GRI 204 Procurement practices 2016 103-1 Management of the topic: Explanation of the material topic P. 38; 109-116; 138 and its Boundary 103-2 Management of the topic: The management approach P. 109-116 and its components 103-3 Management of the topic: Evaluation of the management approach P. 109-116 204-1 Proportion of spending on local suppliers P. 114-116 GRI 205 Anti-corruption 2016 103-1 Management of the topic: Explanation of the material topic P. 34-35; 38; 138 and its Boundary 103-2 Management of the topic: The management approach P. 34-35 and its components Territory 140 |
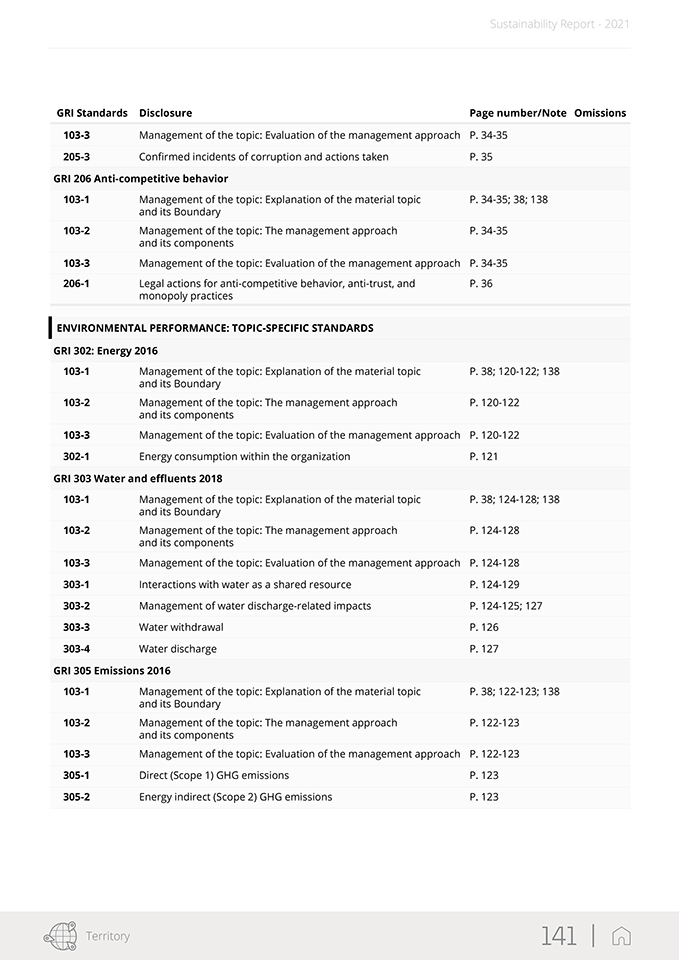
Sustainability Report—2021 GRI Standards Disclosure Page number/Note Omissions 103-3 Management of the topic: Evaluation of the management approach P. 34-35 205-3 Confirmed incidents of corruption and actions taken P. 35 GRI 206 Anti-competitive behavior 103-1 Management of the topic: Explanation of the material topic P. 34-35; 38; 138 and its Boundary 103-2 Management of the topic: The management approach P. 34-35 and its components 103-3 Management of the topic: Evaluation of the management approach P. 34-35 206-1 Legal actions for anti-competitive behavior, anti-trust, and P. 36 monopoly practices ENVIRONMENTAL PERFORMANCE: TOPIC-SPECIFIC STANDARDS GRI 302: Energy 2016 103-1 Management of the topic: Explanation of the material topic P. 38; 120-122; 138 and its Boundary 103-2 Management of the topic: The management approach P. 120-122 and its components 103-3 Management of the topic: Evaluation of the management approach P. 120-122 302-1 Energy consumption within the organization P. 121 GRI 303 Water and effluents 2018 103-1 Management of the topic: Explanation of the material topic P. 38; 124-128; 138 and its Boundary 103-2 Management of the topic: The management approach P. 124-128 and its components 103-3 Management of the topic: Evaluation of the management approach P. 124-128 303-1 Interactions with water as a shared resource P. 124-129 303-2 Management of water discharge-related impacts P. 124-125; 127 303-3 Water withdrawal P. 126 303-4 Water discharge P. 127 GRI 305 Emissions 2016 103-1 Management of the topic: Explanation of the material topic P. 38; 122-123; 138 and its Boundary 103-2 Management of the topic: The management approach P. 122-123 and its components 103-3 Management of the topic: Evaluation of the management approach P. 122-123 305-1 Direct (Scope 1) GHG emissions P. 123 305-2 Energy indirect (Scope 2) GHG emissions P. 123 Territory 141 |
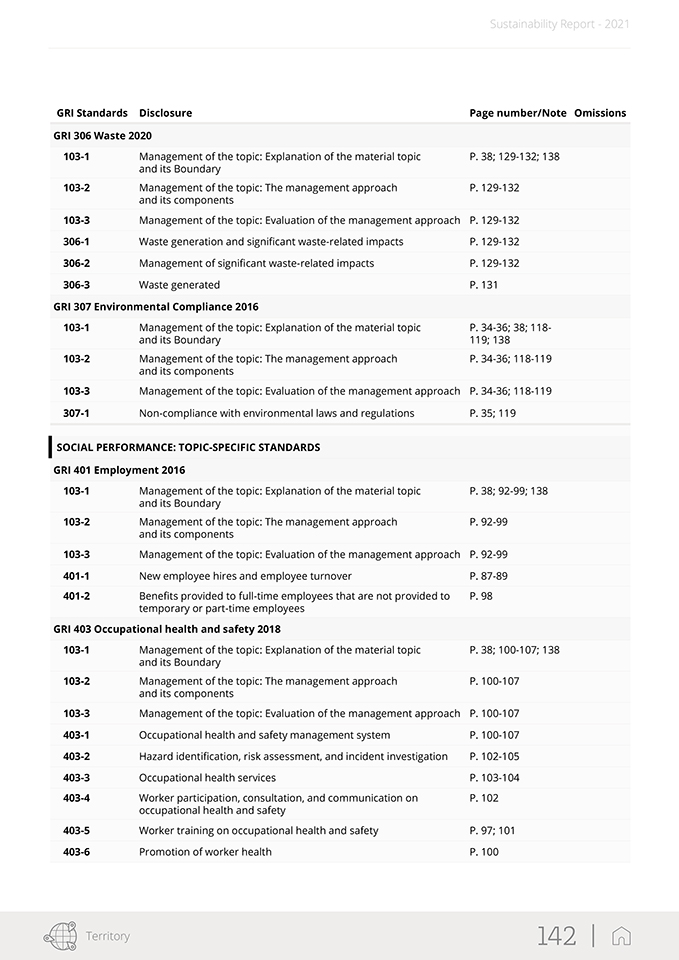
Sustainability Report—2021 GRI Standards Disclosure Page number/Note Omissions GRI 306 Waste 2020 103-1 Management of the topic: Explanation of the material topic P. 38; 129-132; 138 and its Boundary 103-2 Management of the topic: The management approach P. 129-132 and its components 103-3 Management of the topic: Evaluation of the management approach P. 129-132 306-1 Waste generation and significant waste-related impacts P. 129-132 306-2 Management of significant waste-related impacts P. 129-132 306-3 Waste generated P. 131 GRI 307 Environmental Compliance 2016 103-1 Management of the topic: Explanation of the material topic P. 34-36; 38; 118-and its Boundary 119; 138 103-2 Management of the topic: The management approach P. 34-36; 118-119 and its components 103-3 Management of the topic: Evaluation of the management approach P. 34-36; 118-119 307-1 Non-compliance with environmental laws and regulations P. 35; 119 SOCIAL PERFORMANCE: TOPIC-SPECIFIC STANDARDS GRI 401 Employment 2016 103-1 Management of the topic: Explanation of the material topic P. 38; 92-99; 138 and its Boundary 103-2 Management of the topic: The management approach P. 92-99 and its components 103-3 Management of the topic: Evaluation of the management approach P. 92-99 401-1 New employee hires and employee turnover P. 87-89 401-2 Benefits provided to full-time employees that are not provided to P. 98 temporary or part-time employees GRI 403 Occupational health and safety 2018 103-1 Management of the topic: Explanation of the material topic P. 38; 100-107; 138 and its Boundary 103-2 Management of the topic: The management approach P. 100-107 and its components 103-3 Management of the topic: Evaluation of the management approach P. 100-107 403-1 Occupational health and safety management system P. 100-107 403-2 Hazard identification, risk assessment, and incident investigation P. 102-105 403-3 Occupational health services P. 103-104 403-4 Worker participation, consultation, and communication on P. 102 occupational health and safety 403-5 Worker training on occupational health and safety P. 97; 101 403-6 Promotion of worker health P. 100 Territory 142 |
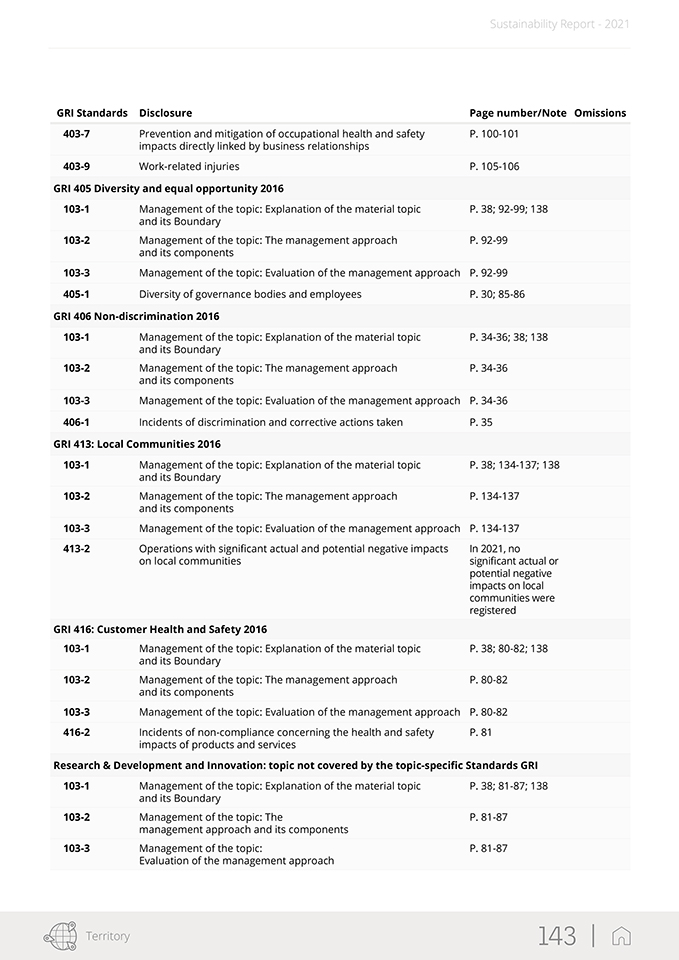
Sustainability Report—2021 GRI Standards Disclosure Page number/Note Omissions 403-7 Prevention and mitigation of occupational health and safety P. 100-101 impacts directly linked by business relationships 403-9 Work-related injuries P. 105-106 GRI 405 Diversity and equal opportunity 2016 103-1 Management of the topic: Explanation of the material topic P. 38; 92-99; 138 and its Boundary 103-2 Management of the topic: The management approach P. 92-99 and its components 103-3 Management of the topic: Evaluation of the management approach P. 92-99 405-1 Diversity of governance bodies and employees P. 30; 85-86 GRI 406 Non-discrimination 2016 103-1 Management of the topic: Explanation of the material topic P. 34-36; 38; 138 and its Boundary 103-2 Management of the topic: The management approach P. 34-36 and its components 103-3 Management of the topic: Evaluation of the management approach P. 34-36 406-1 Incidents of discrimination and corrective actions taken P. 35 GRI 413: Local Communities 2016 103-1 Management of the topic: Explanation of the material topic P. 38; 134-137; 138 and its Boundary 103-2 Management of the topic: The management approach P. 134-137 and its components 103-3 Management of the topic: Evaluation of the management approach P. 134-137 413-2 Operations with significant actual and potential negative impacts In 2021, no on local communities significant actual or potential negative impacts on local communities were registered GRI 416: Customer Health and Safety 2016 103-1 Management of the topic: Explanation of the material topic P. 38; 80-82; 138 and its Boundary 103-2 Management of the topic: The management approach P. 80-82 and its components 103-3 Management of the topic: Evaluation of the management approach P. 80-82 416-2 Incidents of non-compliance concerning the health and safety P. 81 impacts of products and services Research & Development and Innovation: topic not covered by the topic-specific Standards GRI 103-1 Management of the topic: Explanation of the material topic P. 38; 81-87; 138 and its Boundary 103-2 Management of the topic: The P. 81-87 management approach and its components 103-3 Management of the topic: P. 81-87 Evaluation of the management approach Territory 143 |

Deloitte &ToucheS.p.A. Via Fratelli Bandiera., 3 31100Treviso Italia Independent Audit Report Deloitte Tel: +39 0422 5875 Fax:+390422 587812 www.cleloitte.it INDEPENDENT AUDITOR’S REPORT ON THE SUSTAINABILITY REPORT To the Board of Directors of Stevanato Group S.p.A. We have carried out a limited assurance engagement on the sustainability report (“Sustainability Report”) of Stevanato Group and its subsidiaries (hereinafter also “Group”) as of December 31, 2021. Responsibility of the Directors for the Sustainability Report The Directors of Stevanato Group S.p.A. are responsible for the preparation of the Sustainability Report n accordance with the “Global Reporting Initiative Sustainability Reporting Standards” established by GRI—Global Reporting Initiative (“GRI Standards”), which they have identified as reporting framework as specified in the “Methodological Note” paragraph of the Sustainability Report. The Directors are also responsible, for such internal control as they determine is necessary to enable the preparation of the Sustainability Report that is free from material misstatement, whether due to fraud or error. The Directors are also responsible for the definition of the Group’s objectives in relation to the sustainability performance, for the identification of the stakeholders and the significant aspects to report. Auditor’s Independence and quality control We have complied with the independence and other ethical requirements of the Code Of Ethics for Professional Accountants issued bythe International Ethics Standards Board for Accountants, which is founded on fundamental principles of integrity, objectivity, professional competence and due care, confidentiality and professional behaviour. Our auditing firm applies International Standardon Quality Control 1 (ISQC Italia 1) and, accordingly, maintains a comprehensive system of quality control including documented policies and procedures regarding compliance with ethical requirements, professional standards and applicable legal and regulatory requirements. Auditor’s responsibility Our responsibility is to express our conclusion based on the procedures performed about the compliance of the Sustainability Report with the GRI Standards. We conducted our work in accordance with the criteria established in the “International Standard on Assurance Engagements ISAE 3000 (Revised) -Assurance Engagements Other than Audits or Reviews of Historical Financial Information/’ (hereinafter “ISAE 3000 Revised”), issued by the International Auditing and Assurance Standards Board (IAASB) for imited assurance engagements. The standard requires that we plan and perform the engagement to obtain limited assurance whether the Sustainability Report is free from material misstatement. Ancona Ban Bergamo Bologna Brescia Caglari Rrenze Genova Milano NapoJi Padcva Parma Roma Torino TreyisoUdineVerona SedeLegale:ViaTortona, 25—20144 Mitano | CapitaleSodale: Euro 10.328.220,00 i.v. Codice Fscale/RegistrodelleImpresedi MilanoMonza BrianzaLcdi n. 03049560166—REA n. MI-1720239 | Partita IVA: IT03049560166 II nomeDelcittesi nfenscea unao pie celle seguenti entta: Deloitte louche Tohmatsu United, unasodetairglesea responsabilita limitata [“DTTL”), le memberfirmaderenti alsuo network e leenfrta a esse correlate. DTTLeciascuna dellesue memberfirm sono entita giuridicamenteseparate e indipendenti tra loro. DTTL (denominate anche “Delocte Global”) non fomisce seivizi a clients.Si invitaaleggere rinforrratiua completa rebtwa alia descrizione deHa stajttura legaledi DelotteTojcheTohmatsu Limited e delle sue memberfirmall’indirizzo www.d a lo’tte.corrV a bout El Iteioirte &Toiiche SpA

Deloitte. 2 Therefore, the procedures performed in a limited assurance engagement are less than those performed in a reasonable assurance engagement in accordance with ISAE 3000 Revised, and, therefore, do not enable us to obtain assurance that we would become aware of all significant matters and events that might be identified in a reasonable assurance engagement. The procedures performed on the Sustainability Report are based on our professional judgement and included inquiries, primarily with Company personnel responsible for the preparation of information included in the Sustainability Report, analysis of documents, recalculations and other procedures aimed to obtain evidence as appropriate. Specifically, we carried out the following procedures: analysis of the process relating to the definition of material aspects disclosed in the Sustainability Report, with reference to the methods used for the identification and prioritization of material aspects for stakeholders and to the internal validation of the process results; comparison between the economic and financial data and information included in the paragraph titled “Key financial results” of the Sustainability Report with those included in the Group’s financial statements; understanding of the processes underlying the origination, recording and management of qualitative and quantitative material information included in the Sustainability Report. In particular, we carried out interviews and discussions with the management of Stevanato Group S.p.A. and with the personnel of Nuova Ompi S.r.l., Spami S.r.L, Balda Medical Gmbh and Ompi N.A.S. de RL de CV and we carried out limited documentary verifications in order to gather information about the processes and procedures which support the collection, aggregation, elaboration and transmittal of non-financial data and information to the department responsible for the preparation of the Sustainability Report. In addition, for material information, taking into consideration the Group’s activities and characteristics: at the Group’s level: with regards to qualitative information included in the Sustainability Report, we carried out interviews and gathered supporting documentation in order to verify its consistency with the available evidence; with regards to quantitative information, we carried out both analytical procedures and limited verifications in order to ensure, on a sample basis, the correct aggregation of data; for Nuova Ompi S.r.l. and Spami S.r.L, which we selected based on their activities and their contribution to the performance indicators at the consolidated level, we carried out remote meetings, during which we met the management and gathered supporting documentation with reference to the correct application of procedures and calculation methods used for the indicators. Territory ]_45
Deloitte 3 Conclusion Based on the work performed, nothing has come to our attention that causes us to believe that the Sustainability Report of Stevanato Group as of December 31, 2021 is not prepared, in all material aspects, in accordance with the GRI Standards as stated in the paragraph “Methodological note” of the Sustainability Report. Other aspects The data for the year ended December 31, 2020, presented for comparative purposes in the Sustainability Report, have not been subject to a limited or to a reasonable assurance engagement. DELOITTE & TOUCHES.p.A. \Barbara Moscardi Partner Treviso, Italy May 3, 2022

Headquarters Global footprint with operating units Via Molinella, 17 and commercial offices in 9 countries. 35017 Piombino Dese Padova, Italy Visit our website to contact us. stevanatogroup.com